The 5,6,7,8-Tetrahydro-2-Naphthoyl-Coenzyme A Reductase Reaction in the Anaerobic Degradation of Naphthalene and Identification of Downstream Metabolites
- PMID: 32444470
- PMCID: PMC7376553
- DOI: 10.1128/AEM.00996-20
The 5,6,7,8-Tetrahydro-2-Naphthoyl-Coenzyme A Reductase Reaction in the Anaerobic Degradation of Naphthalene and Identification of Downstream Metabolites
Abstract
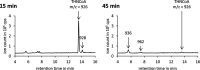
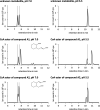
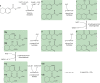
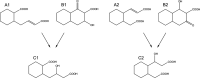
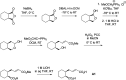
Anaerobic degradation of polycyclic aromatic hydrocarbons has been investigated mostly with naphthalene as a model compound. Naphthalene degradation by sulfate-reducing bacteria proceeds via carboxylation to 2-naphthoic acid, formation of a coenzyme A thioester, and subsequent reduction to 5,6,7,8-tetrahydro-2-naphthoyl-coenzyme A (THNCoA), which is further reduced to hexahydro-2-naphthoyl-CoA (HHNCoA) by tetrahydronaphthoyl-CoA reductase (THNCoA reductase), an enzyme similar to class I benzoyl-CoA reductases. When analyzing THNCoA reductase assays with crude cell extracts and NADH as electron donor via liquid chromatography-mass spectrometry (LC-MS), scanning for putative metabolites, we found that small amounts of the product of an HHNCoA hydratase were formed in the assays, but the downstream conversion by an NAD+-dependent β-hydroxyacyl-CoA dehydrogenase was prevented by the excess of NADH in those assays. Experiments with alternative electron donors indicated that 2-oxoglutarate can serve as an indirect electron donor for the THNCoA-reducing system via a 2-oxoglutarate:ferredoxin oxidoreductase. With 2-oxoglutarate as electron donor, THNCoA was completely converted and further metabolites resulting from subsequent β-oxidation-like reactions and hydrolytic ring cleavage were detected. These metabolites indicate a downstream pathway with water addition to HHNCoA and ring fission via a hydrolase acting on a β'-hydroxy-β-oxo-decahydro-2-naphthoyl-CoA intermediate. Formation of the downstream intermediate cis-2-carboxycyclohexylacetyl-CoA, which is the substrate for the previously described lower degradation pathway leading to the central metabolism, completes the anaerobic degradation pathway of naphthalene.IMPORTANCE Anaerobic degradation of polycyclic aromatic hydrocarbons is poorly investigated despite its significance in anoxic sediments. Using alternative electron donors for the 5,6,7,8-tetrahydro-2-naphthoyl-CoA reductase reaction, we observed intermediary metabolites of anaerobic naphthalene degradation via in vitro enzyme assays with cell extracts of anaerobic naphthalene degraders. The identified metabolites provide evidence that ring reduction terminates at the stage of hexahydro-2-naphthoyl-CoA and a sequence of β-oxidation-like degradation reactions starts with a hydratase acting on this intermediate. The final product of this reaction sequence was identified as cis-2-carboxycyclohexylacetyl-CoA, a compound for which a further downstream degradation pathway has recently been published (P. Weyrauch, A. V. Zaytsev, S. Stephan, L. Kocks, et al., Environ Microbiol 19:2819-2830, 2017, https://doi.org/10.1111/1462-2920.13806). Our study reveals the first ring-cleaving reaction in the anaerobic naphthalene degradation pathway. It closes the gap between the reduction of the first ring of 2-naphthoyl-CoA by 2-napthoyl-CoA reductase and the lower degradation pathway starting from cis-2-carboxycyclohexylacetyl-CoA, where the second ring cleavage takes place.
Keywords: THNCoA reductase; anaerobic catabolic pathways; naphthalene; polycyclic aromatic hydrocarbons.
Copyright © 2020 American Society for Microbiology.
Figures





Similar articles
-
ATP-dependent/-independent enzymatic ring reductions involved in the anaerobic catabolism of naphthalene.Environ Microbiol. 2013 Jun;15(6):1832-41. doi: 10.1111/1462-2920.12076. Epub 2013 Jan 22. Environ Microbiol. 2013. PMID: 23336264
-
Combined genomic and proteomic approaches identify gene clusters involved in anaerobic 2-methylnaphthalene degradation in the sulfate-reducing enrichment culture N47.J Bacteriol. 2010 Jan;192(1):295-306. doi: 10.1128/JB.00874-09. J Bacteriol. 2010. PMID: 19854898 Free PMC article.
-
Two distinct old yellow enzymes are involved in naphthyl ring reduction during anaerobic naphthalene degradation.Mol Microbiol. 2015 Jan;95(2):162-72. doi: 10.1111/mmi.12875. Epub 2014 Dec 22. Mol Microbiol. 2015. PMID: 25424741
-
Anaerobic degradation of polycyclic aromatic hydrocarbons.Appl Environ Microbiol. 2025 Apr 23;91(4):e0226824. doi: 10.1128/aem.02268-24. Epub 2025 Apr 2. Appl Environ Microbiol. 2025. PMID: 40172203 Free PMC article. Review.
-
Anaerobic degradation of polycyclic aromatic hydrocarbons.FEMS Microbiol Ecol. 2004 Jul 1;49(1):27-36. doi: 10.1016/j.femsec.2004.02.019. FEMS Microbiol Ecol. 2004. PMID: 19712381 Review.
Cited by
-
Potential Toxicity Risk Assessment and Priority Control Strategy for PAHs Metabolism and Transformation Behaviors in the Environment.Int J Environ Res Public Health. 2022 Sep 2;19(17):10972. doi: 10.3390/ijerph191710972. Int J Environ Res Public Health. 2022. PMID: 36078713 Free PMC article.
-
Chemoorganoautotrophic lifestyle of the anaerobic enrichment culture N47 growing on naphthalene.Commun Biol. 2025 Jun 4;8(1):856. doi: 10.1038/s42003-025-08172-y. Commun Biol. 2025. PMID: 40467871 Free PMC article.
-
The ever-expanding limits of enzyme catalysis and biodegradation: polyaromatic, polychlorinated, polyfluorinated, and polymeric compounds.Biochem J. 2020 Aug 14;477(15):2875-2891. doi: 10.1042/BCJ20190720. Biochem J. 2020. PMID: 32797216 Free PMC article. Review.
-
Characterization of 2-phenanthroyl-CoA reductase, an ATP-independent type III aryl-CoA reductase involved in anaerobic phenanthrene degradation.Appl Environ Microbiol. 2025 May 21;91(5):e0016625. doi: 10.1128/aem.00166-25. Epub 2025 Apr 17. Appl Environ Microbiol. 2025. PMID: 40243319 Free PMC article.
-
A CoA-Transferase and Acyl-CoA Dehydrogenase Convert 2-(Carboxymethyl)cyclohexane-1-Carboxyl-CoA During Anaerobic Naphthalene Degradation.Environ Microbiol. 2024 Dec;26(12):e70013. doi: 10.1111/1462-2920.70013. Environ Microbiol. 2024. PMID: 39702997 Free PMC article.
References
-
- Wilkes H, Schwarzbauer J. 2010. Hydrocarbons: an introduction to structure, physico-chemical properties and natural occurrence, p 1–48. In Timmis KN. (ed), Handbook of hydrocarbon and lipid microbiology. Springer, Berlin, Germany.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

