The 6-4 photoproduct is the trigger of UV-induced replication blockage and ATR activation
- PMID: 32444488
- PMCID: PMC7293618
- DOI: 10.1073/pnas.1917196117
The 6-4 photoproduct is the trigger of UV-induced replication blockage and ATR activation
Abstract
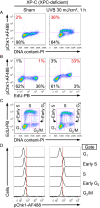
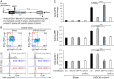
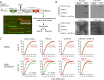
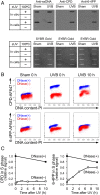
The most prevalent human carcinogen is sunlight-associated ultraviolet (UV), a physiologic dose of which generates thousands of DNA lesions per cell, mostly of two types: cyclobutane pyrimidine dimers (CPDs) and 6-4 photoproducts (6-4PPs). It has not been possible, in living cells, to precisely characterize the respective contributions of these two lesion types to the signals that regulate cell cycle progression, DNA replication, and cell survival. Here we coupled multiparameter flow cytometry with lesion-specific photolyases that eliminate either CPDs or 6-4PPs and determined their respective contributions to DNA damage responses. Strikingly, only 6-4PP lesions activated the ATR-Chk1 DNA damage response pathway. Mechanistically, 6-4PPs, but not CPDs, impeded DNA replication across the genome as revealed by microfluidic-assisted replication track analysis. Furthermore, single-stranded DNA accumulated preferentially at 6-4PPs during DNA replication, indicating selective and prolonged replication blockage at 6-4PPs. These findings suggest that 6-4PPs, although eightfold fewer in number than CPDs, are the trigger for UV-induced DNA damage responses.
Keywords: 6-4PP; CPD; Chk1; DNA damage response; DNA replication.
Conflict of interest statement
The authors declare no competing interest.
Figures

Similar articles
-
CPDs and 6-4PPs play different roles in UV-induced cell death in normal and NER-deficient human cells.DNA Repair (Amst). 2008 Feb 1;7(2):303-12. doi: 10.1016/j.dnarep.2007.11.003. Epub 2007 Dec 21. DNA Repair (Amst). 2008. PMID: 18096446
-
Powerful skin cancer protection by a CPD-photolyase transgene.Curr Biol. 2005 Jan 26;15(2):105-15. doi: 10.1016/j.cub.2005.01.001. Curr Biol. 2005. PMID: 15668165
-
Temperature-dependent formation and photorepair of DNA damage induced by UV-B radiation in suspension-cultured tobacco cells.J Photochem Photobiol B. 2002 Feb;66(1):67-72. doi: 10.1016/s1011-1344(01)00277-9. J Photochem Photobiol B. 2002. PMID: 11849985
-
Photolyases: capturing the light to battle skin cancer.Future Oncol. 2006 Apr;2(2):191-9. doi: 10.2217/14796694.2.2.191. Future Oncol. 2006. PMID: 16563088 Review.
-
Quantitation and visualization of ultraviolet-induced DNA damage using specific antibodies: application to pigment cell biology.Pigment Cell Res. 2001 Apr;14(2):94-102. doi: 10.1034/j.1600-0749.2001.140204.x. Pigment Cell Res. 2001. PMID: 11310797 Review.
Cited by
-
Investigation on possible resistance of Candida auris to irradiation with UVC (254 nm).MicroPubl Biol. 2025 Mar 20;2025:10.17912/micropub.biology.001421. doi: 10.17912/micropub.biology.001421. eCollection 2025. MicroPubl Biol. 2025. PMID: 40191443 Free PMC article.
-
Medicinal Plant Extracts Targeting UV-Induced Skin Damage: Molecular Mechanisms and Therapeutic Potential.Int J Mol Sci. 2025 Mar 4;26(5):2278. doi: 10.3390/ijms26052278. Int J Mol Sci. 2025. PMID: 40076896 Free PMC article. Review.
-
Human CST complex restricts excessive PrimPol repriming upon UV induced replication stress by suppressing p21.Nucleic Acids Res. 2024 Apr 24;52(7):3778-3793. doi: 10.1093/nar/gkae078. Nucleic Acids Res. 2024. PMID: 38348929 Free PMC article.
-
Phototherapy and DNA Damage: A Systematic Review.J Clin Aesthet Dermatol. 2023 Jun;16(6):55-58. J Clin Aesthet Dermatol. 2023. PMID: 37361361 Free PMC article.
-
Post-replicative lesion processing limits DNA damage-induced mutagenesis.Nucleic Acids Res. 2025 Mar 20;53(6):gkaf198. doi: 10.1093/nar/gkaf198. Nucleic Acids Res. 2025. PMID: 40114379 Free PMC article.
References
-
- Rogers H. W., Weinstock M. A., Feldman S. R., Coldiron B. M., Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the U.S. population, 2012. JAMA Dermatol. 151, 1081–1086 (2015). - PubMed
-
- Jans J. et al. ., Powerful skin cancer protection by a CPD-photolyase transgene. Curr. Biol. 15, 105–115 (2005). - PubMed
-
- Sancar A., DNA excision repair. Annu. Rev. Biochem. 65, 43–81 (1996). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

