Tolcapone Treatment for Cognitive and Behavioral Symptoms in Behavioral Variant Frontotemporal Dementia: A Placebo-Controlled Crossover Study
- PMID: 32444540
- PMCID: PMC10131251
- DOI: 10.3233/JAD-191265
Tolcapone Treatment for Cognitive and Behavioral Symptoms in Behavioral Variant Frontotemporal Dementia: A Placebo-Controlled Crossover Study
Abstract
Background: There are currently no disease-targeted treatments for cognitive or behavioral symptoms in patients with behavioral variant frontotemporal dementia (bvFTD).
Objective: To determine the effect of tolcapone, a specific inhibitor of Catechol-O-Methyltransferase (COMT), in patients with bvFTD.
Methods: In this randomized, double-blind, placebo-controlled, cross-over study at two study sites, we examined the effect of tolcapone on 28 adult outpatients with bvFTD. The primary outcome was reaction time on the N-back cognitive test. As an imaging outcome, we examined differences in the resting blood oxygen level dependent (BOLD) functional magnetic resonance imaging (fMRI) signal intensity between subjects on placebo versus tolcapone performing the N-back test. Secondary outcomes included measures of cognitive performance and behavioral disturbance using the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS), Neuropsychiatric Inventory-Questionnaire (NPI-Q), and Clinical Global Impressions scale (CGI).
Results: Tolcapone was well tolerated and no patients dropped out. The most frequent treatment-related adverse event during tolcapone treatment was elevated liver enzymes (21%). There were no significant differences between tolcapone treatment and placebo in the primary or imaging outcomes. However, there were significant differences between RBANS total scores (p < 0.01), NPI-Q total scores (p = 0.04), and CGI total scores (p = 0.035) between treatment conditions which were driven by differences between baseline and tolcapone conditions. Further, there was a trend toward significance between tolcapone and placebo on the CGI (p = 0.078).
Conclusions: Further study of COMT inhibition and related approaches with longer duration of treatment and larger sample sizes in frontotemporal lobar degeneration-spectrum disorders may be warranted.
Keywords: COMT; dopamine; frontotemporal dementia; tolcapone; treatment.
Figures


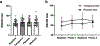
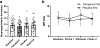

References
-
- Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, van Swieten JC, Seelaar H, Dopper EG, Onyike CU, Hillis AE, Josephs KA, Boeve BF, Kertesz A, Seeley WW, Rankin KP, Johnson JK, Gorno-Tempini ML, Rosen H, Prioleau-Latham CE, Lee A, Kipps CM, Lillo P, Piguet O, Rohrer JD, Rossor MN, Warren JD, Fox NC, Galasko D, Salmon DP, Black SE, Mesulam M, Weintraub S, Dickerson BC, Diehl-Schmid J, Pasquier F, Deramecourt V, Lebert F, Pijnenburg Y, Chow TW, Manes F, Grafman J, Cappa SF, Freedman M, Grossman M, Miller BL (2011) Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 134, 2456–2477. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

