Retrospective Analysis of Eculizumab in Patients with Acetylcholine Receptor Antibody-Negative Myasthenia Gravis: A Case Series
- PMID: 32444555
- PMCID: PMC7369065
- DOI: 10.3233/JND-190464
Retrospective Analysis of Eculizumab in Patients with Acetylcholine Receptor Antibody-Negative Myasthenia Gravis: A Case Series
Abstract
Background: The role of the complement cascade in acetylcholine receptor antibody-negative (AChR-) myasthenia gravis (MG) is unclear.
Objective: To assess the efficacy and tolerability of eculizumab (terminal complement inhibitor) in patients with AChR-MG.
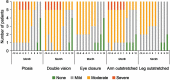
Methods: Retrospective chart review of data from six patients treated for 12 months with eculizumab for treatment-refractory, AChR-(by radioimmunoassay) generalized MG (gMG). The eculizumab dose was 900 mg/week for 4 weeks then 1200 mg every 2 weeks. Outcome measures were Myasthenia Gravis-Activities of Daily Living (MG-ADL) scores, number of exacerbations, and qualitative physical assessments based on selected items of the Quantitative Myasthenia Gravis evaluation (ptosis, double vision, eye closure, duration of ability to stretch out limbs).
Results: All patients were female (mean age, 50.8 years). In the 12 months before eculizumab initiation, all measures were relatively stable. After its initiation, clinically meaningful reductions (≥2 points) in total MG-ADL scores were observed before or at 5 months and were maintained to Month 12 in all patients; mean (standard deviation [SD]) scores were 11.3 (0.9) and 5.0 (0.9), respectively. There was also a reduction in the mean (SD) number of exacerbations per patient, from 2.8 (1.2) to 0.3 (0.5) in the 12 months before and after eculizumab initiation, respectively. Physical assessment ratings were improved in all patients. Adverse events were reported in four patients, but all were mild and none were treatment-related.
Conclusions: This small retrospective analysis provides preliminary evidence for the efficacy of eculizumab in treatment-refractory gMG that was AChR-according to radioimmunoassay. Larger, more robust studies are warranted to evaluate this further.
Keywords: ACh receptors; Myasthenia gravis; activities of daily living; complement inactivating agents; corticosteroids; neuromuscular junction.
Conflict of interest statement
Sorabh Datta and Shivangi Singh have no conflicts of interest; Raghav Govindarajan serves on the advisory boards of Alexion Pharmaceuticals, argenx Pharmaceuticals, Mitsubishi Tanabe Pharmaceuticals, and Catalyst Pharmaceuticals, and has received research support from Alexion Pharmaceuticals, Ra Pharmaceuticals, Strongbridge Pharmaceuticals, The American Academy of Neurology, AMARC Enterprises/Poly-MVA, Dysimmune Foundation and InfuCare. He has received speaker’s honoraria from Alexion Pharmaceuticals, Mitsubishi Tanabe Pharmaceuticals, Catalyst Pharmaceuticals, and Muscular Dystrophy Association, and publication honorarium from Springer Publishing, USA.
Figures




Similar articles
-
Clinical Experience with Eculizumab in Treatment-Refractory Acetylcholine Receptor Antibody-Positive Generalized Myasthenia Gravis.J Neuromuscul Dis. 2021;8(2):287-294. doi: 10.3233/JND-200584. J Neuromuscul Dis. 2021. PMID: 33325394 Free PMC article.
-
Eculizumab in Adolescent Patients With Refractory Generalized Myasthenia Gravis: A Phase 3, Open-Label, Multicenter Study.Pediatr Neurol. 2024 Jul;156:198-207. doi: 10.1016/j.pediatrneurol.2024.04.020. Epub 2024 Apr 26. Pediatr Neurol. 2024. PMID: 38810600 Clinical Trial.
-
Eculizumab: A Review in Generalized Myasthenia Gravis.Drugs. 2018 Mar;78(3):367-376. doi: 10.1007/s40265-018-0875-9. Drugs. 2018. PMID: 29435915 Free PMC article. Review.
-
Safety and efficacy of eculizumab in anti-acetylcholine receptor antibody-positive refractory generalised myasthenia gravis (REGAIN): a phase 3, randomised, double-blind, placebo-controlled, multicentre study.Lancet Neurol. 2017 Dec;16(12):976-986. doi: 10.1016/S1474-4422(17)30369-1. Epub 2017 Oct 20. Lancet Neurol. 2017. PMID: 29066163 Clinical Trial.
-
Eculizumab treatment for myasthenia gravis subgroups: 2021 update.J Neuroimmunol. 2022 Jan 15;362:577767. doi: 10.1016/j.jneuroim.2021.577767. Epub 2021 Nov 18. J Neuroimmunol. 2022. PMID: 34823117 Review.
Cited by
-
Practical Management for Use of Eculizumab in the Treatment of Severe, Refractory, Non-Thymomatous, AChR + Generalized Myasthenia Gravis: A Systematic Review.Ther Clin Risk Manag. 2022 Jul 12;18:699-719. doi: 10.2147/TCRM.S266031. eCollection 2022. Ther Clin Risk Manag. 2022. PMID: 35855752 Free PMC article. Review.
-
Eculizumab in refractory myasthenia gravis: a real-world single-center experience.Neurol Sci. 2025 Feb;46(2):951-959. doi: 10.1007/s10072-024-07861-6. Epub 2024 Nov 4. Neurol Sci. 2025. PMID: 39495373
-
Adverse Side Effects Associated with Corticosteroid Therapy: A Study in 39 Patients with Generalized Myasthenia Gravis.Med Sci Monit. 2021 Oct 28;27:e933296. doi: 10.12659/MSM.933296. Med Sci Monit. 2021. PMID: 34707081 Free PMC article.
-
Utilization of MG-ADL in myasthenia gravis clinical research and care.Muscle Nerve. 2022 Jun;65(6):630-639. doi: 10.1002/mus.27476. Epub 2022 Jan 6. Muscle Nerve. 2022. PMID: 34989427 Free PMC article. Review.
-
Role of complement in myasthenia gravis.Front Neurol. 2023 Oct 5;14:1277596. doi: 10.3389/fneur.2023.1277596. eCollection 2023. Front Neurol. 2023. PMID: 37869140 Free PMC article. Review.
References
-
- Berrih-Aknin S, Frenkian-Cuvelier M, Eymard B. Diagnostic and clinical classification of autoimmune myasthenia gravis. J Autoimmun. 2014;48-49:143–8. [PubMed: 24530233]. - PubMed
-
- Gilhus NE. Myasthenia gravis. N Engl J Med. 2016;375(26):2570–81. [PubMed: 28029925]. - PubMed
-
- Gilhus NE, Skeie GO, Romi F, Lazaridis K, Zisimopoulou P, Tzartos S. Myasthenia gravis –autoantibody characteristics and their implications for therapy. Nat Rev Neurol. 2016;12(5):259–68. [PubMed: 27103470]. - PubMed
-
- Gilhus NE, Verschuuren JJ. Myasthenia gravis: Subgroup classification and therapeutic strategies. Lancet Neurol. 2015;14(10):1023–36. [PubMed: 26376969]. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

