Fracture toughness of a metal-organic framework glass
- PMID: 32444664
- PMCID: PMC7244719
- DOI: 10.1038/s41467-020-16382-7
Fracture toughness of a metal-organic framework glass
Abstract
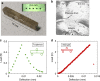
Metal-organic framework glasses feature unique thermal, structural, and chemical properties compared to traditional metallic, organic, and oxide glasses. So far, there is a lack of knowledge of their mechanical properties, especially toughness and strength, owing to the challenge in preparing large bulk glass samples for mechanical testing. However, a recently developed melting method enables fabrication of large bulk glass samples (>25 mm3) from zeolitic imidazolate frameworks. Here, fracture toughness (KIc) of a representative glass, namely ZIF-62 glass (Zn(C3H3N2)1.75(C7H5N2)0.25), is measured using single-edge precracked beam method and simulated using reactive molecular dynamics. KIc is determined to be ~0.1 MPa m0.5, which is even lower than that of brittle oxide glasses due to the preferential breakage of the weak coordinative bonds (Zn-N). The glass is found to exhibit an anomalous brittle-to-ductile transition behavior, considering its low fracture surface energy despite similar Poisson's ratio to that of many ductile metallic and organic glasses.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Observation of indentation-induced shear bands in a metal-organic framework glass.Proc Natl Acad Sci U S A. 2020 May 12;117(19):10149-10154. doi: 10.1073/pnas.2000916117. Epub 2020 Apr 27. Proc Natl Acad Sci U S A. 2020. PMID: 32341165 Free PMC article.
-
Bond switching is responsible for nanoductility in zeolitic imidazolate framework glasses.Dalton Trans. 2021 May 11;50(18):6126-6132. doi: 10.1039/d1dt00096a. Dalton Trans. 2021. PMID: 33973604
-
Revisiting the Dependence of Poisson's Ratio on Liquid Fragility and Atomic Packing Density in Oxide Glasses.Materials (Basel). 2019 Jul 31;12(15):2439. doi: 10.3390/ma12152439. Materials (Basel). 2019. PMID: 31370218 Free PMC article.
-
Poisson's Ratio of Glasses, Ceramics, and Crystals.Materials (Basel). 2024 Jan 7;17(2):300. doi: 10.3390/ma17020300. Materials (Basel). 2024. PMID: 38255468 Free PMC article. Review.
-
Recent advances in bulk metallic glasses for biomedical applications.Acta Biomater. 2016 May;36:1-20. doi: 10.1016/j.actbio.2016.03.047. Epub 2016 Apr 1. Acta Biomater. 2016. PMID: 27045349 Review.
Cited by
-
Precise control over gas-transporting channels in zeolitic imidazolate framework glasses.Nat Mater. 2024 Feb;23(2):262-270. doi: 10.1038/s41563-023-01738-3. Epub 2023 Dec 20. Nat Mater. 2024. PMID: 38123813 Free PMC article.
-
Continuous structure modification of metal-organic framework glasses via halide salts.Nat Commun. 2025 Jul 30;16(1):7001. doi: 10.1038/s41467-025-62143-9. Nat Commun. 2025. PMID: 40739141 Free PMC article.
-
A Short Review of Advances in MOF Glass Membranes for Gas Adsorption and Separation.Membranes (Basel). 2024 Apr 25;14(5):99. doi: 10.3390/membranes14050099. Membranes (Basel). 2024. PMID: 38786934 Free PMC article. Review.
-
Micro-optical elements from optical-quality ZIF-62 hybrid glasses by hot imprinting.Nat Commun. 2024 Jun 13;15(1):5079. doi: 10.1038/s41467-024-49428-1. Nat Commun. 2024. PMID: 38871703 Free PMC article.
-
Melting of hybrid organic-inorganic perovskites.Nat Chem. 2021 Aug;13(8):778-785. doi: 10.1038/s41557-021-00681-7. Epub 2021 May 10. Nat Chem. 2021. PMID: 33972755
References
-
- Mason JA, et al. Methane storage in flexible metal-organic frameworks with intrinsic thermal management. Nature. 2015;527:357–361. - PubMed
-
- Su Z, et al. Shock Wave Chemistry in a metal-organic framework. J. Am. Chem. Soc. 2017;139:4619–4622. - PubMed
-
- Yoon JW, et al. Selective nitrogen capture by porous hybrid materials containing accessible transition metal ion sites. Nat. Mater. 2017;16:526–531. - PubMed
-
- Mondloch JE, et al. Destruction of chemical warfare agents using metal-organic frameworks. Nat. Mater. 2015;14:512–516. - PubMed
-
- Wu T, Feng X, Elsaidi SK, Thallapally PK, Carreon MA. Zeolitic imidazolate framework-8 (ZIF-8) membranes for Kr/Xe separation. Ind. Eng. Chem. Res. 2017;56:1682–1686.

