The strategic combination of trastuzumab emtansine with oncolytic rhabdoviruses leads to therapeutic synergy
- PMID: 32444806
- PMCID: PMC7244474
- DOI: 10.1038/s42003-020-0972-7
The strategic combination of trastuzumab emtansine with oncolytic rhabdoviruses leads to therapeutic synergy
Abstract
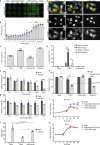
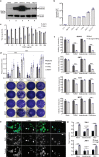
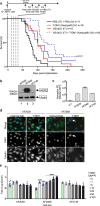
We have demonstrated that microtubule destabilizing agents (MDAs) can sensitize tumors to oncolytic vesicular stomatitis virus (VSVΔ51) in various preclinical models of cancer. The clinically approved T-DM1 (Kadcyla®) is an antibody-drug conjugate consisting of HER2-targeting trastuzumab linked to the potent MDA and maytansine derivative DM1. We reveal that combining T-DM1 with VSVΔ51 leads to increased viral spread and tumor killing in trastuzumab-binding, VSVΔ51-resistant cancer cells. In vivo, co-treatment of VSVΔ51 and T-DM1 increased overall survival in HER2-overexpressing, but trastuzumab-refractory, JIMT1 human breast cancer xenografts compared to monotherapies. Furthermore, viral spread in cultured HER2+ human ovarian cancer patient-derived ascites samples was enhanced by the combination of VSVΔ51 and T-DM1. Our data using the clinically approved Kadcyla® in combination with VSVΔ51 demonstrates proof of concept that targeted delivery of a viral-sensitizing molecule using an antibody-drug conjugate can enhance oncolytic virus activity and provides rationale for translation of this approach.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Verri E, et al. HER2/neu oncoprotein overexpression in epithelial ovarian cancer: evaluation of its prevalence and prognostic significance. Clin. Study Oncol. 2005;68:154–161. - PubMed
-
- Palle, J. et al. Human epidermal growth factor receptor 2 (HER2) in advanced gastric cancer: current knowledge and future perspectives. Drugs80, 401–415 (2020). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

