Management of patients with multiple myeloma in the era of COVID-19 pandemic: a consensus paper from the European Myeloma Network (EMN)
- PMID: 32444866
- PMCID: PMC7244257
- DOI: 10.1038/s41375-020-0876-z
Management of patients with multiple myeloma in the era of COVID-19 pandemic: a consensus paper from the European Myeloma Network (EMN)
Abstract
Patients with multiple myeloma (MM) seem to be at increased risk for more severe COVID-19 infection and associated complications due to their immunocompromised state, the older age and comorbidities. The European Myeloma Network has provided an expert consensus statement in order to guide therapeutic decisions in the era of the COVID-19 pandemic. Patient education for personal hygiene and social distancing measures, along with treatment individualization, telemedicine and continuous surveillance for early diagnosis of COVID-19 are essential. In countries or local communities where COVID-19 infection is widely spread, MM patients should have a PCR test of nasopharyngeal swab for SARS-CoV-2 before hospital admission, starting a new treatment line, cell apheresis or ASCT in order to avoid ward or community spread and infections. Oral agent-based regimens should be considered, especially for the elderly and frail patients with standard risk disease, whereas de-intensified regimens for dexamethasone, bortezomib, carfilzomib and daratumumab should be used based on patient risk and response. Treatment initiation should not be postponed for patients with end organ damage, myeloma emergencies and aggressive relapses. Autologous (and especially allogeneic) transplantation should be delayed and extended induction should be administered, especially in standard risk patients and those with adequate MM response to induction. Watchful waiting should be considered for standard risk relapsed patients with low tumor burden, and slow biochemical relapses. The conduction of clinical trials should continue with appropriate adaptations to the current circumstances. Patients with MM and symptomatic COVID-19 disease should interrupt anti-myeloma treatment until recovery. For patients with positive PCR test for SARS-CoV-2, but with no symptoms for COVID-19, a 14-day quarantine should be considered if myeloma-related events allow the delay of treatment. The need for surveillance for drug interactions due to polypharmacy is highlighted. The participation in international COVID-19 cancer registries is greatly encouraged.
Conflict of interest statement
ET declares honoraria from BMS, Janssen, Celgene, Takeda, Genesis Pharma, Amgen and Novartis; research funding from Janssen, Amgen, Takeda and Genesis Pharma; ME declares no competing financial interests for this paper; GC declares Honoraria: Amgen, Bristol-Myers Squibb, Celgene, Janssen, Sanofi, Karyopharm and GSK; Research funding: Celgene, Janssen, Takeda; FG declares honoraria from BMS, Janssen, Celgene, Takeda, Amgen; advisory board from Janssen, Amgen, Takeda, Adaptive, Celgene, Oncopeptides, Roche, Abbvie, GSK; M-VM has received honoraria from lectures and boards from: Janssen, Celgene, Amgen, Takeda, Abbvie, GSK, Adaptive, Roche, Seattle Genetics; IN-S declares no competing financial interests for this paper; NWCJD has received research support from Janssen Pharmaceuticals, AMGEN, Celgene, Novartis, and BMS, serves in advisory boards for Janssen Pharmaceuticals, AMGEN, Celgene, BMS, Takeda, Roche, Novartis, Bayer, and Servier; HA-L declares no competing financial interests for this paper; RH has received research funding from Janssen, Amgen, Celgene, BMS, Novartis and Takeda, serves in advisory boards for Janssen, Amgen, Celgene, AbbVie, BMS, Novartis, PharmaMar and Takeda, and has received honoraria from Janssen, Amgen, Celgene, BMS, Pharma Mar and Takeda; AJV declares no competing financial interests for this paper; HL has received research funding from Takeda, Amgen, serves in Speaker’s Bureau/Advisory Boards for Takeda, Amgen, Janssen, Celgene, Sanofi, Seattle Genetics and Bristol-Meyers. SZ has received research funding from Takeda and Janssen and serves in advisory boards for Celgene, Takeda, Janssen, Sanofi and Oncopeptides; PM declares honorarium form Celgene, Janssen, Takeda, Amgen and Abbvie; HE has received honoraria and declares consultancy for Janssen, Celgene, BMS, Amgen, Novartis and Takeda, and received research support from Janssen, Celgene, BMS, Amgen. MB has received honoraria from Sanofi, Celgene, Amgen, Janssen, Novartis, Bristol-Myers Squibb, and AbbVie; has received research funding from Sanofi, Celgene, Amgen, Janssen, Novartis, Bristol-Myers Squibb, and Mundipharma; JSM declares consultancy for Amgen, Bristol-Myers Squibb, Celgene, Janssen, MSD, Novartis, GSK Takeda, Sanofi, and Roche; MAD declares consultancy and honoraria from Janssen, Celgene, Takeda, Amgen and BMS; PS declares research funding from Amgen, Celgene, Janssen, Karyopharm, SkylineDx and Takeda and honoraria from Amgen, BMS, Celgene, Janssen, Karyopharm and Takeda.
Figures
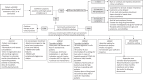
References
-
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a Report of 72314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020. 10.1001/jama.2020.2648. [Epub ahead of print]. - PubMed
-
- Dai M, Liu D, Liu M, Zhou F, Li G, Chen Z, et al. Patients with cancer appear more vulnerable to SARS-COV-2: a multi-center study during the COVID-19 outbreak. Cancer Discov. 2020. 10.1158/2159-8290.CD-20-0422. [Epub ahead of print]. - PMC - PubMed

