A systematic review and meta-analyses of pregnancy and fetal outcomes in women with multiple sclerosis: a contribution from the IMI2 ConcePTION project
- PMID: 32444984
- PMCID: PMC7419441
- DOI: 10.1007/s00415-020-09913-1
A systematic review and meta-analyses of pregnancy and fetal outcomes in women with multiple sclerosis: a contribution from the IMI2 ConcePTION project
Abstract
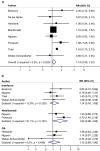
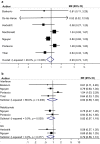
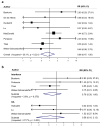
Neurologists managing women with Multiple Sclerosis (MS) need information about the safety of disease modifying drugs (DMDs) during pregnancy. However, this knowledge is limited. The present study aims to summarize previous studies by performing a systematic review and meta-analyses. The terms "multiple sclerosis" combined with DMDs of interest and a broad profile for pregnancy terms were used to search Embase and Medline databases to identify relevant studies published from January 2000 to July 2019.1260 studies were identified and ten studies met our inclusion criteria. Pooled risk ratios (RR) of pregnancy and birth outcomes in pregnancies exposed to DMDs compared to those not exposed were calculated using a random effects model. For spontaneous abortion RR = 1.14, 95% CI 0.99-1.32, for preterm births RR = 0.93, 95% CI 0.72-1.21 and for major congenital malformations RR = 0.86, 95% CI 0.47-1.56. The most common major congenital malformations reported in MS patients exposed to MS drugs were atrial septal defect (ASD) (N = 4), polydactyly (N = 4) and club foot (N = 3), which are among the most prevalent birth defects observed in the general population. In conclusion, interferons, glatiramer acetate or natalizumab, do not appear to increase the risk for spontaneous abortions, pre-term birth or major congenital malformations. There were very few patients included that were exposed to fingolimod, azathioprine and rituximab; therefore, these results cannot be generalized across drugs. Future studies including internal comparators are needed to enable treating physicians and their patients to decide on the best treatment options.
Keywords: Congenital malformations; Multiple sclerosis; Pregnancy; Preterm; Spontaneous abortions.
Conflict of interest statement
SLL and YG are an employee of Novartis, MS is employee of Merck, and MT is contracted by Novartis AG.
Figures
References
-
- Multiple Sclerosis International Federation (2013) Atlas of MS 2013. Mapping Multiple Sclerosis Around the World. https://www.msif.org/wp-content/uploads/2014/09/Atlas-of-MS.pdf. Accessed Jun 2019
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

