Etrolizumab for the Treatment of Ulcerative Colitis and Crohn's Disease: An Overview of the Phase 3 Clinical Program
- PMID: 32445184
- PMCID: PMC7467434
- DOI: 10.1007/s12325-020-01366-2
Etrolizumab for the Treatment of Ulcerative Colitis and Crohn's Disease: An Overview of the Phase 3 Clinical Program
Abstract
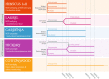
Introduction: Etrolizumab is a next-generation anti-integrin with dual action that targets two pathways of inflammation in the gut. A robust phase 3 clinical program in ulcerative colitis (UC) and Crohn's disease is ongoing and will evaluate the efficacy and safety of etrolizumab in well-defined patient populations in rigorous trials that include direct head-to-head comparisons against approved anti-tumor necrosis factor alpha agents (anti-TNF). The etrolizumab phase 3 clinical program consists of six randomized controlled trials (RCTs; UC: HIBISCUS I and II, GARDENIA, LAUREL, HICKORY; Crohn's disease: BERGAMOT) and two open-label extension trials (OLEs; UC: COTTONWOOD; Crohn's disease: JUNIPER) evaluating patients with moderately to severely active UC or Crohn's disease.
Methods: In the UC RCTs, patients are randomly assigned according to each protocol to receive etrolizumab, adalimumab, infliximab, or placebo. In BERGAMOT, patients are randomly assigned to receive etrolizumab 105 mg, etrolizumab 210 mg, or placebo. The primary outcomes for the UC RCTs are Mayo Clinic score-based clinical response, remission, and clinical remission; for BERGAMOT, the co-primary outcomes are clinical remission (based on abdominal pain and stool frequency) and endoscopic improvement (based on the Simple Endoscopic Score for Crohn's disease). The OLEs will primarily assess long-term efficacy and safety. Secondary and exploratory endpoints include endoscopy, histology, quality of life, and biomarkers at various timepoints.
Discussion: The etrolizumab phase 3 clinical program is the largest and most comprehensive in inflammatory bowel disease, enrolling more than 3000 patients. The program explores both induction and maintenance regimens. HIBISCUS I and II and GARDENIA are among the first head-to-head trials in UC against an anti-TNF and are the first registrational trials making that comparison. This program will also help address unanswered clinical questions on evaluation of treatment effects and treatment selection across a range of patients with varying treatment histories using an extensive repository of patient samples and data.
Trial registration: ClinicalTrials.gov: HIBISCUS I (NCT02163759), HIBISCUS II (NCT02171429), GARDENIA (NCT02136069), LAUREL (NCT02165215), HICKORY (NCT02100696), COTTONWOOD (NCT02118584), BERGAMOT (NCT02394028), JUNIPER (NCT02403323).
Keywords: Anti-integrin; Crohn’s disease; Etrolizumab; Inflammatory bowel disease; Ulcerative colitis.
Figures


References
-
- Stein RB, Hanauer SB. Comparative tolerability of treatments for inflammatory bowel disease. Drug Saf. 2000;23(5):429–448. - PubMed
-
- Ford AC, Peyrin-Biroulet L. Opportunistic infections with anti-tumor necrosis factor-alpha therapy in inflammatory bowel disease: meta-analysis of randomized controlled trials. Am J Gastroenterol. 2013;108(8):1268–1276. - PubMed
-
- Sands BE, Sandborn WJ, Panaccione R, et al. Ustekinumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2019;381(13):1201–1214. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

