The pharmacokinetics, efficacy, and safety of a novel selective-dose cannabis inhaler in patients with chronic pain: A randomized, double-blinded, placebo-controlled trial
- PMID: 32445190
- PMCID: PMC7496774
- DOI: 10.1002/ejp.1605
The pharmacokinetics, efficacy, and safety of a novel selective-dose cannabis inhaler in patients with chronic pain: A randomized, double-blinded, placebo-controlled trial
Abstract
Background: Precise cannabis treatment dosing remains a major challenge, leading to physicians' reluctance to prescribe medical cannabis.
Objective: To test the pharmacokinetics, analgesic effect, cognitive performance and safety effects of an innovative medical device that enables the delivery of inhaled therapeutic doses of Δ9 -Tetrahydrocannabinol (THC) in patients with chronic pain.
Methods: In a randomized, three-arms, double-blinded, placebo-controlled, cross-over trial, 27 patients received a single inhalation of Δ9 -THC: 0.5mg, 1mg, or a placebo. Δ9 -THC plasma levels were measured at baseline and up to 150-min post-inhalation. Pain intensity and safety parameters were recorded on a 10-cm visual analogue scale (VAS) at pre-defined time points. The cognitive performance was evaluated using the selective sub-tests of the Cambridge Neuropsychological Test Automated Battery (CANTAB).
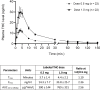
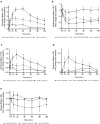
Results: Following inhalation of 0.5 mg or 1mg, Δ9 -THC plasma Cmax ± SD were 14.3 ± 7.7 and 33.8 ± 25.7 ng/ml. Tmax ± SD were 3.7 ± 1.4 and 4.4 ± 2.1 min, and AUC0 → infinity ±SD were 300 ± 144 and 769 ± 331 ng*min/ml, respectively. Both doses, but not the placebo, demonstrated a significant reduction in pain intensity compared with baseline and remained stable for 150-min. The 1-mg dose showed a significant pain decrease compared to the placebo. Adverse events were mostly mild and resolved spontaneously. There was no evidence of consistent impairments in cognitive performance.
Conclusion: This feasibility trial demonstrated that a metered-dose cannabis inhaler delivered precise and low THC doses, produced a dose-dependent and safe analgesic effect in patients with neuropathic pain/ complex-regional pain syndrome (CRPS). Thus, it enables individualization of medical cannabis regimens that can be evaluated pharmacokinetically and pharmacodynamically by accepted pharmaceutical models.
Significance: Evidence suggests that cannabis-based medicines are an effective treatment for chronic pain in adults. The pharmacokinetics of THC varies as a function of its route of administration. Pulmonary assimilation of inhaled THC causes rapid onset of analgesia. However, currently used routes of cannabinoids delivery provide unknown doses, making it impossible to implement a pharmaceutical standard treatment plan. A novel selective-dose cannabis inhaler delivers significantly low and precise doses of THC, thus allowing the administration of inhaled cannabis-based medicines according to high pharmaceutical standards. These low doses of THC can produce safe and effective analgesia in patients with chronic pain.
© 2020 The Authors. European Journal of Pain published by John Wiley & Sons Ltd on behalf of European Pain Federation - EFIC ®.
Conflict of interest statement
Dr. Almog is a consultant for Syqe Medical; Prof. Aharon‐Peretz reports no disclosures; Dr. Hayon, Mrs. Ogintz, Mrs. Abalia, and Mr. Lupo are employees of Syqe Medical; Dr. Vulfsons and Prof. Eisenberg received research support from Syqe Medical.
Figures


References
-
- Abrams, D. I. , Vizoso, H. P. , Shade, S. B. , Jay, C. , Kelly, M. E. , & Benowitz, N. L. (2007). Vaporization as a smokeless cannabis delivery system: A pilot study. Clinical Pharmacology and Therapeutics, 82, 572–578. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

