Remdesivir for the Treatment of Covid-19 - Final Report
- PMID: 32445440
- PMCID: PMC7262788
- DOI: 10.1056/NEJMoa2007764
Remdesivir for the Treatment of Covid-19 - Final Report
Abstract
Background: Although several therapeutic agents have been evaluated for the treatment of coronavirus disease 2019 (Covid-19), no antiviral agents have yet been shown to be efficacious.
Methods: We conducted a double-blind, randomized, placebo-controlled trial of intravenous remdesivir in adults who were hospitalized with Covid-19 and had evidence of lower respiratory tract infection. Patients were randomly assigned to receive either remdesivir (200 mg loading dose on day 1, followed by 100 mg daily for up to 9 additional days) or placebo for up to 10 days. The primary outcome was the time to recovery, defined by either discharge from the hospital or hospitalization for infection-control purposes only.
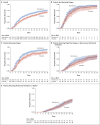
Results: A total of 1062 patients underwent randomization (with 541 assigned to remdesivir and 521 to placebo). Those who received remdesivir had a median recovery time of 10 days (95% confidence interval [CI], 9 to 11), as compared with 15 days (95% CI, 13 to 18) among those who received placebo (rate ratio for recovery, 1.29; 95% CI, 1.12 to 1.49; P<0.001, by a log-rank test). In an analysis that used a proportional-odds model with an eight-category ordinal scale, the patients who received remdesivir were found to be more likely than those who received placebo to have clinical improvement at day 15 (odds ratio, 1.5; 95% CI, 1.2 to 1.9, after adjustment for actual disease severity). The Kaplan-Meier estimates of mortality were 6.7% with remdesivir and 11.9% with placebo by day 15 and 11.4% with remdesivir and 15.2% with placebo by day 29 (hazard ratio, 0.73; 95% CI, 0.52 to 1.03). Serious adverse events were reported in 131 of the 532 patients who received remdesivir (24.6%) and in 163 of the 516 patients who received placebo (31.6%).
Conclusions: Our data show that remdesivir was superior to placebo in shortening the time to recovery in adults who were hospitalized with Covid-19 and had evidence of lower respiratory tract infection. (Funded by the National Institute of Allergy and Infectious Diseases and others; ACTT-1 ClinicalTrials.gov number, NCT04280705.).
Copyright © 2020 Massachusetts Medical Society.
Figures

Comment in
-
Remdesivir - An Important First Step.N Engl J Med. 2020 Nov 5;383(19):1886-1887. doi: 10.1056/NEJMe2018715. Epub 2020 May 27. N Engl J Med. 2020. PMID: 32459913 Free PMC article. No abstract available.
-
[Remdesivir til behandling af COVID-19-pneumoni].Ugeskr Laeger. 2020 Jun 8;182(24):V205031. Ugeskr Laeger. 2020. PMID: 32515337 Danish. No abstract available.
-
Remdesivir for the Treatment of Covid-19 - Preliminary Report.N Engl J Med. 2020 Sep 3;383(10):992. doi: 10.1056/NEJMc2022236. Epub 2020 Jul 10. N Engl J Med. 2020. PMID: 32649074 No abstract available.
-
Remdesivir for the Treatment of Covid-19 - Preliminary Report.N Engl J Med. 2020 Sep 3;383(10):992-993. doi: 10.1056/NEJMc2022236. Epub 2020 Jul 10. N Engl J Med. 2020. PMID: 32649075 No abstract available.
-
Remdesivir for the Treatment of Covid-19 - Preliminary Report.N Engl J Med. 2020 Sep 3;383(10):993. doi: 10.1056/NEJMc2022236. Epub 2020 Jul 10. N Engl J Med. 2020. PMID: 32649076 No abstract available.
-
Remdesivir for the Treatment of Covid-19 - Preliminary Report.N Engl J Med. 2020 Sep 3;383(10):993-994. doi: 10.1056/NEJMc2022236. Epub 2020 Jul 10. N Engl J Med. 2020. PMID: 32649077 No abstract available.
References
-
- Borba MGS, Val FFA, Sampaio VS, et al. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: a randomized clinical trial. JAMA Netw Open 2020;3(4):e208857-e208857. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- UM1AI148576/US/United States/United States
- I01 BX003714/BX/BLRD VA/United States
- UM1AI148685/US/United States/United States
- UM1AI148452/US/United States/United States
- UM1 AI148685/AI/NIAID NIH HHS/United States
- UM1AI148684/US/United States/United States
- UM1 AI148573/AI/NIAID NIH HHS/United States
- UM1 AI148684/AI/NIAID NIH HHS/United States
- 211153/Z/18/Z/WT_/Wellcome Trust/United Kingdom
- HHSN261201500003I/CA/NCI NIH HHS/United States
- MC_UU_12023/22/MRC_/Medical Research Council/United Kingdom
- HHSN261200800001E/US/United States/United States
- R38 AI140299/AI/NIAID NIH HHS/United States
- UM1 AI148689/AI/NIAID NIH HHS/United States
- UM1 AI148450/AI/NIAID NIH HHS/United States
- U54 AG062334/AG/NIA NIH HHS/United States
- UM1AI148573/US/United States/United States
- UM1 AI148575/AI/NIAID NIH HHS/United States
- UM1 AI148576/AI/NIAID NIH HHS/United States
- UM1AI148575/US/United States/United States
- HHSN261201500003C/CA/NCI NIH HHS/United States
- UM1AI148689/US/United States/United States
- UM1 AI148452/AI/NIAID NIH HHS/United States
- HHSN261201500003I/US/United States/United States
- UM1AI148450/US/United States/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
