Somatic cell conversion to a germ cell lineage: A violation or a revelation?
- PMID: 32445519
- PMCID: PMC7680723
- DOI: 10.1002/jez.b.22952
Somatic cell conversion to a germ cell lineage: A violation or a revelation?
Abstract
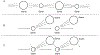
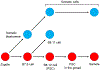
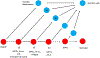
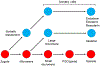
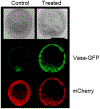
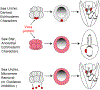
The germline is unique and immortal (or at least its genome is). It is able to perform unique jobs (meiosis) and is selected for genetic changes. Part of being this special also means that entry into the germline club is restricted and cells of the soma are always left out. However, the recent evidence from multiple animals now suggests that somatic cells may join the club and become germline cells in an animal when the original germline is removed. This "violation" may have garnered acceptance by the observation that iPScells, originating experimentally from somatic cells of an adult, can form reproductively successful eggs and sperm, all in vitro. Each of the genes and their functions used to induce pluripotentiality are found normally in the cell and the in vitro conditions to direct germline commitment replicate conditions in vivo. Here, we discuss evidence from three different animals: an ascidian, a segmented worm, and a sea urchin; and that the cells of a somatic cell lineage can convert into the germline in vivo. We discuss the consequences of such transitions and provide thoughts as how this process may have equal precision to the original germline formation of an embryo.
Keywords: cell fate conversion; germ cell; germline; soma.
© 2020 Wiley Periodicals LLC.
Figures






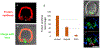
Similar articles
-
Soma-germ cell interactions in Caenorhabditis elegans: multiple events of hermaphrodite germline development require the somatic sheath and spermathecal lineages.Dev Biol. 1997 Jan 15;181(2):121-43. doi: 10.1006/dbio.1996.8429. Dev Biol. 1997. PMID: 9013925
-
H3K27me3 suppresses sister-lineage somatic gene expression in late embryonic germline cells of the ascidian, Halocynthia roretzi.Dev Biol. 2020 Apr 15;460(2):200-214. doi: 10.1016/j.ydbio.2019.12.017. Epub 2020 Jan 2. Dev Biol. 2020. PMID: 31904374
-
Regeneration of the germline in the annelid Capitella teleta.Dev Biol. 2018 Aug 15;440(2):74-87. doi: 10.1016/j.ydbio.2018.05.004. Epub 2018 May 19. Dev Biol. 2018. PMID: 29758179
-
Germline cell formation and gonad regeneration in solitary and colonial ascidians.Dev Dyn. 2011 Feb;240(2):299-308. doi: 10.1002/dvdy.22542. Epub 2011 Jan 11. Dev Dyn. 2011. PMID: 21246647 Review.
-
Germ cell specification.Adv Exp Med Biol. 2013;757:17-39. doi: 10.1007/978-1-4614-4015-4_2. Adv Exp Med Biol. 2013. PMID: 22872473 Free PMC article. Review.
Cited by
-
Regeneration in the Segmented Annelid Capitella teleta.Genes (Basel). 2021 Nov 8;12(11):1769. doi: 10.3390/genes12111769. Genes (Basel). 2021. PMID: 34828375 Free PMC article. Review.
-
Functional annotation of a hugely expanded nanos repertoire in Lytechinus variegatus, the green sea urchin.Mol Reprod Dev. 2023 May;90(5):310-322. doi: 10.1002/mrd.23684. Epub 2023 Apr 11. Mol Reprod Dev. 2023. PMID: 37039283 Free PMC article.
-
Molding immortality from a plastic germline.Curr Opin Cell Biol. 2021 Dec;73:1-8. doi: 10.1016/j.ceb.2021.04.010. Epub 2021 Jun 3. Curr Opin Cell Biol. 2021. PMID: 34091218 Free PMC article. Review.
-
Inheritance of somatic mutations by animal offspring.Sci Adv. 2022 Sep 2;8(35):eabn0707. doi: 10.1126/sciadv.abn0707. Epub 2022 Aug 31. Sci Adv. 2022. PMID: 36044584 Free PMC article.
-
Annelids as models of germ cell and gonad regeneration.J Exp Zool B Mol Dev Evol. 2024 May;342(3):126-143. doi: 10.1002/jez.b.23233. Epub 2023 Dec 11. J Exp Zool B Mol Dev Evol. 2024. PMID: 38078561 Free PMC article. Review.
References
-
- Buss L (1987). The Evolution of Individuality: Princeton University Press.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

