Management of von Willebrand disease with a factor VIII-poor von Willebrand factor concentrate: Results from a prospective observational post-marketing study
- PMID: 32445594
- PMCID: PMC7496521
- DOI: 10.1111/jth.14928
Management of von Willebrand disease with a factor VIII-poor von Willebrand factor concentrate: Results from a prospective observational post-marketing study
Abstract
Background: A triple-secured plasma-derived von Willebrand factor (pdVWF) almost devoid of factor VIII (FVIII):WILFACTIN® , was approved in France in 2003, and then in other countries for the treatment of patients with von Willebrand disease (VWD).
Objective: To investigate long-term safety and efficacy of the product in real-life over the first 5 post-approval years.
Patients/methods: This prospective, observational, national post-marketing study (PMS) enrolled patients of all ages and VWD types. Patients were observed for up to 3 years and treated for one or more occasions. Efficacy was assessed for each major event. Breakthrough bleeding rate 3 days post-infusion and annualized bleeding rate (ABR) were also evaluated for long-term prophylaxis.
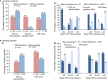
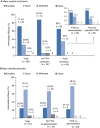
Results: Overall, 155 of 174 patients enrolled from 31 centers were eligible for efficacy assessment. Most patients (76.8%) were severely affected (VWF:RCo ≤ 15 IU/dL). They were treated for 743 bleeds and 140 surgeries including childbirth. Efficacy outcomes were excellent/good for 98.2% of 56 major surgeries and 94.0% of 67 major bleeds. Approximately 75% of 49 major mucosal bleeds were effectively managed without FVIII co-administration. In 32 patients receiving prophylaxis, breakthrough bleeding occurred in 1.5% of infusions and median ABR was 1.0 for 20 patients treated ≥ 12 months. Excellent tolerability was confirmed with no safety concerns. No thrombotic events were observed.
Conclusions: Results from this PMS increase the clinical experience of a FVIII-poor pdVWF in patients of all ages and VWD types including those with thrombotic risk factors and emphasize that giving FVIII is not always mandatory to effectively treat patients with severe VWD.
Keywords: clinical trial; factor VIII; post-marketing; von Willebrand disease; von Willebrand factor.
© 2020 The Authors. Journal of Thrombosis and Haemostasis published by Wiley Periodicals LLC on behalf of International Society on Thrombosis and Haemostasis.
Conflict of interest statement
This work was funded by LFB, Les Ulis, France. A. Harroche, P. Chamouni have no conflicts of interest to declare. J. Goudemand received support for attending scientific meetings and honoraria from Baxalta‐Shire, Bayer Healthcare, CSL Behring, LFB, Novo‐Nordisk, Roche‐Chugai, and Sobi. S. Claeyssens received support for attending scientific meetings from Baxalta/Shire, Bayer, LFB, Novo‐Nordisk, Octapharma, Sobi, and honoraria from Octapharma and Sobi. N. Itzhar‐Baïkian received travel fees from LFB, Shire‐Takeda, and CSL‐Behring. D. Desprez received research support for her institution and travel fees from Sobi. C. Négrier reports grants and personal fees from Baxalta/Shire, CSL Behring, Octapharma, Sobi, and personal fees from Alnylam Pharmaceuticals, Bayer, LFB, Novo Nordisk, Pfizer, Roche. All are outside the submitted work. H. Chambost has received support for attending scientific meetings and honoraria from Baxalta‐Shire, Bayer Healthcare, CSL Behring, LFB, Novo Nordisk, Octapharma, Pfizer, Roche‐Chugaï, and Sobi; has been an investigator in studies sponsored by Baxter, Bayer, Biomarin, Bioverativ, CSL Behring, LFB, and Octapharma; and has received research support from CSL Behring, LFB, Novo Nordisk, Octapharma, and Sobi (none of these relate to the present study). S. Susen received research support for her institution from LFB and CSL‐Behring, and performed consultancy for LFB, Roche, Sobi, Takeda: fees go to the institution. She is co‐founder and owner of Laelaps Therapeutics. A. Borel‐Derlon received research support for her institution from CSL Behring, Octapharma, Novo Nordisk, Baxalta, Shire: Consultant for Sobi, LFB. F. Bridey and C. Henriet are employees of LFB.
Figures
References
-
- Leebeek FW, Eikenboom JC. Von Willebrand’s disease. N Engl J Med. 2016;375:2067‐2080. - PubMed
-
- Castaman G, Federici AB, Rodeghiero F, Mannucci PM. Von Willebrand’s disease in the year 2003: towards the complete identification of gene defects for correct diagnosis and treatment. Haematologica. 2003;88(1):94‐108. - PubMed
-
- Lenting PJ, Casari C, Christophe OD, Denis CV. von Willebrand factor: the old, the new and the unknown. J Thromb Haemost. 2012;10:2428‐2437. - PubMed
-
- Sadler JE, Budde U, Eikenboom JC, et al. Working Party on von Willebrand Disease Classification. Update on the pathophysiology and classification of von Willebrand disease: a report of the Subcommittee on von Willebrand Factor. J Thromb Haemost. 2006;4:2103‐2114. - PubMed
-
- Rodeghiero F, Castaman G, Dini E. Epidemiological investigation of the prevalence of von Willebrand’s disease. Blood. 1987;69:454‐459. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

