Structural optimization of HPMA copolymer-based dexamethasone prodrug for improved treatment of inflammatory arthritis
- PMID: 32445658
- PMCID: PMC7429277
- DOI: 10.1016/j.jconrel.2020.05.028
Structural optimization of HPMA copolymer-based dexamethasone prodrug for improved treatment of inflammatory arthritis
Abstract
Despite their notorious adverse effects, glucocorticoids (GC, potent anti-inflammatory drugs) are used extensively in clinical management of rheumatoid arthritis (RA) and other chronic inflammatory diseases. To achieve a sustained therapeutic efficacy and reduced toxicities, macromolecular GC prodrugs have been developed with promising outcomes for the treatment of RA. Fine-tuning the activation kinetics of these prodrugs may further improve their therapeutic efficacy and minimize the off-target adverse effects. To assess the feasibility of this strategy, five different dexamethasone (Dex, a potent GC)-containing monomers with distinctively different linker chemistries were designed, synthesized, and copolymerized with N-(2-hydroxypropyl) methacrylamide (HPMA) to obtain 5 macromolecular Dex prodrugs. Their Dex releasing rates were analyzed in vitro and shown to display a wide spectrum of activation kinetics. Their therapeutic efficacy and preliminary toxicology profiles were assessed and compared in vivo in an adjuvant-induced arthritis (AA) rat model in order to identify the ideal prodrug design for the most effective and safe treatment of inflammatory arthritis. The in vivo data demonstrated that the C3 hydrazone linker-containing prodrug design was the most effective in preserving joint structural integrity. The results from this study suggest that the design and screening of different activation mechanisms may help to identify macromolecular prodrugs with the most potent therapeutic efficacy and safety for the management of inflammatory arthritis.
Keywords: Controlled release; Dexamethasone; HPMA copolymer; Inflammation; Prodrug; Rheumatoid arthritis.
Published by Elsevier B.V.
Figures




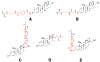
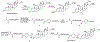
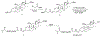

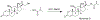
References
-
- Gabriel SE, The epidemiology of rheumatoid arthritis, Rheumatic Disease Clinics of North America 27(2) (2001) 269–281. - PubMed
-
- Zhang W, Anis AH, The economic burden of rheumatoid arthritis: beyond health care costs, Clin Rheumatol 30 Suppl 1 (2011) S25–32. - PubMed
-
- Control C.f.D., Prevention, National and state medical expenditures and lost earnings attributable to arthritis and other rheumatic conditions--United States, 2003, MMWR. Morbidity and mortality weekly report 56(1) (2007) 4. - PubMed
-
- van den Hoven JM, Van Tomme SR, Metselaar JM, Nuijen B, Beijnen JH, Storm G, Liposomal drug formulations in the treatment of rheumatoid arthritis, Mol Pharm 8(4) (2011) 1002–15. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

