Association of maternal thyroid function with birthweight: a systematic review and individual-participant data meta-analysis
- PMID: 32445737
- PMCID: PMC8168324
- DOI: 10.1016/S2213-8587(20)30061-9
Association of maternal thyroid function with birthweight: a systematic review and individual-participant data meta-analysis
Abstract
Background: Adequate transplacental passage of maternal thyroid hormone is important for normal fetal growth and development. Maternal overt hypothyroidism and hyperthyroidism are associated with low birthweight, but important knowledge gaps remain regarding the effect of subclinical thyroid function test abnormalities on birthweight-both in general and during the late second and third trimester of pregnancy. The aim of this study was to examine associations of maternal thyroid function with birthweight.
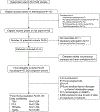
Methods: In this systematic review and individual-participant data meta-analysis, we searched MEDLINE (Ovid), Embase, Web of Science, the Cochrane Central Register of Controlled Trials, and Google Scholar from inception to Oct 15, 2019, for prospective cohort studies with data on maternal thyroid function during pregnancy and birthweight, and we issued open invitations to identify study authors to join the Consortium on Thyroid and Pregnancy. We excluded participants with multiple pregnancies, in-vitro fertilisation, pre-existing thyroid disease or thyroid medication usage, miscarriages, and stillbirths. The main outcomes assessed were small for gestational age (SGA) neonates, large for gestational age neonates, and newborn birthweight. We analysed individual-participant data using mixed-effects regression models adjusting for maternal age, BMI, ethnicity, smoking, parity, gestational age at blood sampling, fetal sex, and gestational age at birth. The study protocol was pre-registered at the International Prospective Register of Systematic Reviews, CRD42016043496.
Findings: We identified 2526 published reports, from which 36 cohorts met the inclusion criteria. The study authors for 15 of these cohorts agreed to participate, and five more unpublished datasets were added, giving a study population of 48 145 mother-child pairs after exclusions, of whom 1275 (3·1%) had subclinical hypothyroidism (increased thyroid stimulating hormone [TSH] with normal free thyroxine [FT4]) and 929 (2·2%) had isolated hypothyroxinaemia (decreased FT4 with normal TSH). Maternal subclinical hypothyroidism was associated with a higher risk of SGA than was euthyroidism (11·8% vs 10·0%; adjusted risk difference 2·43%, 95% CI 0·43 to 4·81; odds ratio [OR] 1·24, 1·04 to 1·48; p=0·015) and lower mean birthweight (mean difference -38 g, -61 to -15; p=0·0015), with a higher effect estimate for measurement in the third trimester than in the first or second. Isolated hypothyroxinaemia was associated with a lower risk of SGA than was euthyroidism (7·3% vs 10·0%, adjusted risk difference -2·91, -4·49 to -0·88; OR 0·70, 0·55 to 0·91; p=0·0073) and higher mean birthweight (mean difference 45 g, 18 to 73; p=0·0012). Each 1 SD increase in maternal TSH concentration was associated with a 6 g lower birthweight (-10 to -2; p=0·0030), with higher effect estimates in women who were thyroid peroxidase antibody positive than for women who were negative (pinteraction=0·10). Each 1 SD increase in FT4 concentration was associated with a 21 g lower birthweight (-25 to -17; p<0·0001), with a higher effect estimate for measurement in the third trimester than the first or second.
Interpretation: Maternal subclinical hypothyroidism in pregnancy is associated with a higher risk of SGA and lower birthweight, whereas isolated hypothyroxinaemia is associated with lower risk of SGA and higher birthweight. There was an inverse, dose-response association of maternal TSH and FT4 (even within the normal range) with birthweight. These results advance our understanding of the complex relationships between maternal thyroid function and fetal outcomes, and they should prompt careful consideration of potential risks and benefits of levothyroxine therapy during pregnancy.
Funding: Netherlands Organization for Scientific Research (grant 401.16.020).
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests
RPP has served as a consultant to Berlin-Chemie AG, GoodLife Fertility BV, and Institut Biochimique SA. TIMK has received personal fees from Berlin Chemie, Goodlife Healthcare, and Quidel. BV has received honoraria from Berlin-Chime. EO has received grants from the National Institutes of Health. TV has received grants from the Netherlands Organization for Health Research and Development. CD has received grants from the Chief Scientist Office (Scotland) and the British Heart Foundation. SMN has received grants from the Chief Scientist Office (Scotland) and reports grants and personal fees from Roche Diagnostics, during the conduct of the study. LC reported serving as a consultant to Pfizer. TM reported serving as a consultant to Abbott Diagnostics and Roche. ENP has received grants from the Sociedad Quimica y Minera de Chile and personal fees from the Institut Biochimique SA. The rest of the authors declare no competing interests.
Figures

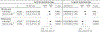
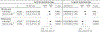

Comment in
-
Maternal thyroid function, levothyroxine, and birthweight-a balancing act.Lancet Diabetes Endocrinol. 2020 Jun;8(6):461-462. doi: 10.1016/S2213-8587(20)30108-X. Lancet Diabetes Endocrinol. 2020. PMID: 32445731 No abstract available.
References
-
- Zimmermann E, Gamborg M, Sorensen TI, Baker JL. Sex Differences in the Association Between Birth Weight and Adult Type 2 Diabetes. Diabetes 2015; 64(12): 4220–5. - PubMed
-
- Zhang Z, Kris-Etherton PM, Hartman TJ. Birth weight and risk factors for cardiovascular disease and type 2 diabetes in US children and adolescents: 10 year results from NHANES. Matern Child Health J 2014; 18(6): 1423–32. - PubMed
-
- Stuart A, Amer-Wahlin I, Persson J, Kallen K. Long-term cardiovascular risk in relation to birth weight and exposure to maternal diabetes mellitus. Int J Cardiol 2013; 168(3): 2653–7. - PubMed
-
- Geserick M, Vogel M, Gausche R, et al. Acceleration of BMI in Early Childhood and Risk of Sustained Obesity. New England Journal of Medicine 2018; 379(14): 1303–12. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

