The Protective Effects of Calcineurin on Pancreatitis in Mice Depend on the Cellular Source
- PMID: 32445858
- PMCID: PMC7502475
- DOI: 10.1053/j.gastro.2020.05.051
The Protective Effects of Calcineurin on Pancreatitis in Mice Depend on the Cellular Source
Abstract
Background & aims: Calcineurin is a ubiquitously expressed central Ca2+-responsive signaling molecule that mediates acute pancreatitis, but little is known about its effects. We compared the effects of calcineurin expression by hematopoietic cells vs pancreas in mouse models of pancreatitis and pancreatitis-associated lung inflammation.
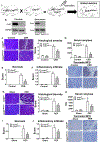
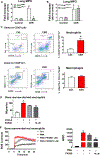
Methods: We performed studies with mice with hematopoietic-specific or pancreas-specific deletion of protein phosphatase 3, regulatory subunit B, alpha isoform (PPP3R1, also called CNB1), in mice with deletion of CNB1 (Cnb1UBC△/△) and in the corresponding controls for each deletion of CNB1. Acute pancreatitis was induced in mice by administration of caerulein or high-pressure infusion of radiocontrast into biliopancreatic ducts; some mice were also given intraductal infusions of an adeno-associated virus vector that expressed nuclear factor of activated T -cells (NFAT)-luciferase into pancreas. Pancreas, bone marrow, liver, kidney, heart, and lung were collected and analyzed by histopathology, immunohistochemistry, and immunoblots; levels of cytokines were measured in serum. Mouse and human primary pancreatic acinar cells were transfected with a vector that expressed NFAT-luciferase and incubated with an agent that blocks interaction of NFAT with calcineurin; cells were analyzed by immunofluorescence. Calcineurin-mediated neutrophil chemotaxis and reactive oxygen species production were measured in neutrophils from mice.
Results: Mice with hematopoietic-specific deletion of CNB1 developed the same level of local pancreatic inflammation as control mice after administration of caerulein or infusion of radiocontrast into biliopancreatic ducts. Cnb1UBC△/△ mice or mice with pancreas-specific deletion of CNB1 developed less severe pancreatitis and reduced pancreatic inflammation after administration of caerulein or infusion of radiocontrast into biliopancreatic ducts compared with control mice. NFAT was activated in pancreas of Swiss Webster mice given caerulein or infusions of radiocontrast into biliopancreatic ducts. Blocking the interaction between calcineurin and NFAT did not reduce pancreatic acinar cell necrosis in response to caerulein or infusions of radiocontrast. Mice with hematopoietic-specific deletion of CNB1 (but not mice with pancreas-specific deletion of CNB1) had reduced infiltration of lung tissues by neutrophils. Neutrophil chemotaxis and production of reactive oxygen species were decreased after incubation with a calcineurin inhibitor.
Conclusions: Hematopoietic and neutrophil expression of calcineurin promotes pancreatitis-associated lung inflammation, whereas pancreatic calcineurin promotes local pancreatic inflammation. The findings indicate that the protective effects of blocking or deleting calcineurin on pancreatitis are mediated by the source of its expression. This information should be used in the development of strategies to inhibit calcineurin for the prevention of pancreatitis and pancreatitis-associated lung inflammation.
Keywords: PEP; calcium signaling; drug development; targeted calcineurin inhibition.
Copyright © 2020 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures





Comment in
-
Cell Signaling of Pancreatic Duct Pressure and Its Role in the Onset of Pancreatitis.Gastroenterology. 2020 Sep;159(3):827-831. doi: 10.1053/j.gastro.2020.07.027. Epub 2020 Jul 18. Gastroenterology. 2020. PMID: 32693183 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

