Exploring the FMN binding site in the mitochondrial outer membrane protein mitoNEET
- PMID: 32445867
- PMCID: PMC7434653
- DOI: 10.1016/j.freeradbiomed.2020.05.004
Exploring the FMN binding site in the mitochondrial outer membrane protein mitoNEET
Abstract
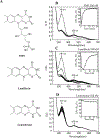
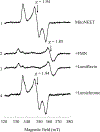
MitoNEET is a mitochondrial outer membrane protein that hosts a redox active [2Fe-2S] cluster in the C-terminal cytosolic domain. Increasing evidence has shown that mitoNEET has an essential role in regulating energy metabolism in human cells. Previously, we reported that the [2Fe-2S] clusters in mitoNEET can be reduced by the reduced flavin mononucleotide (FMNH2) and oxidized by oxygen or ubiquinone-2, suggesting that mitoNEET may act as a novel redox enzyme catalyzing electron transfer from FMNH2 to oxygen or ubiquinone. Here, we explore the FMN binding site in mitoNEET by using FMN analogs and find that lumiflavin, like FMN, at nanomolar concentrations can mediate the redox transition of the mitoNEET [2Fe-2S] clusters in the presence of flavin reductase and NADH (100 μM) under aerobic conditions. The electron paramagnetic resonance (EPR) measurements show that both FMN and lumiflavin can dramatically change the EPR spectrum of the reduced mitoNEET [2Fe-2S] clusters and form a covalently bound complex with mitoNEET under blue light exposure, suggesting that FMN/lumiflavin has specific interactions with the [2Fe-2S] clusters in mitoNEET. In contrast, lumichrome, another FMN analog, fails to mediate the redox transition of the mitoNEET [2Fe-2S] clusters and has no effect on the EPR spectrum of the reduced mitoNEET [2Fe-2S] clusters under blue light exposure. Instead, lumichrome can effectively inhibit the FMNH2-mediated reduction of the mitoNEET [2Fe-2S] clusters, indicating that lumichrome may act as a potential inhibitor to block the electron transfer activity of mitoNEET.
Keywords: Electron transfer activity; FMN; Lumichrome; Lumiflavin; MitoNEET; Mitochondria.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare no competing interests.
Figures




References
-
- Colca JR; McDonald WG; Waldon DJ; Leone JW; Lull JM; Bannow CA; Lund ET; Mathews WR Identification of a novel mitochondrial protein (“mitoNEET”) cross-linked specifically by a thiazolidinedione photoprobe. Am. J. Physiol. Endocrinol. Metab 286:E252–E260; 2004. - PubMed
-
- Tamir S; Paddock ML; Darash-Yahana-Baram M; Holt SH; Sohn YS; Agranat L; Michaeli D; Stofleth JT; Lipper CH; Morcos F; Cabantchik IZ; Onuchic JN; Jennings PA; Mittler R; Nechushtai R Structure-function analysis of NEET proteins uncovers their role as key regulators of iron and ROS homeostasis in health and disease. Biochim. Biophys. Acta 1853:1294–1315; 2015. - PubMed
-
- Sohn YS; Tamir S; Song L; Michaeli D; Matouk I; Conlan AR; Harir Y; Holt SH; Shulaev V; Paddock ML; Hochberg A; Cabanchick IZ; Onuchic JN; Jennings PA; Nechushtai R; Mittler R NAF-1 and mitoNEET are central to human breast cancer proliferation by maintaining mitochondrial homeostasis and promoting tumor growth. Proc. Natl. Acad. Sci. U. S. A 110:14676–14681; 2013. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

