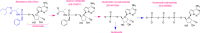Remdesivir: Review of Pharmacology, Pre-clinical Data, and Emerging Clinical Experience for COVID-19
- PMID: 32446287
- PMCID: PMC7283864
- DOI: 10.1002/phar.2429
Remdesivir: Review of Pharmacology, Pre-clinical Data, and Emerging Clinical Experience for COVID-19
Abstract
The global pandemic of novel coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has created an urgent need for effective antivirals. Remdesivir (formerly GS-5734) is a nucleoside analogue pro-drug currently being evaluated in COVID-19 clinical trials. Its unique structural features allow high concentrations of the active triphosphate metabolite to be delivered intracellularly and it evades proofreading to successfully inhibit viral RNA synthesis. In pre-clinical models, remdesivir has demonstrated potent antiviral activity against diverse human and zoonotic β-coronaviruses, including SARS-CoV-2. In this article, we critically review available data on remdesivir with an emphasis on biochemistry, pharmacology, pharmacokinetics, and in vitro activity against coronaviruses as well as clinical experience and current progress in COVID-19 clinical trials.
Keywords: COVID-19; GS-5734; Remdesivir; SARS-CoV-2; coronavirus; severe acute respiratory syndrome.
© 2020 Pharmacotherapy Publications, Inc.
Figures
References
-
- Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID‐19): a review. JAMA 2020;323:1824–36 - PubMed
-
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020;323:1239. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous