Improvement in Function after Lasmiditan Treatment: Post Hoc Analysis of Data from Phase 3 Studies
- PMID: 32447545
- PMCID: PMC7606429
- DOI: 10.1007/s40120-020-00185-5
Improvement in Function after Lasmiditan Treatment: Post Hoc Analysis of Data from Phase 3 Studies
Abstract
Introduction: Migraine is associated with substantial functional impairment and affects many aspects of daily life.
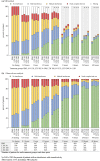
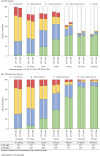
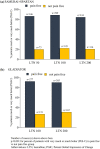
Methods: Using data from SAMURAI and SPARTAN (double-blind, placebo-controlled, phase 3 studies) and GLADIATOR (an open-label, phase 3 study enrolling patients who had completed SAMURAI or SPARTAN), we assessed the effects of lasmiditan on migraine-related functional disability at multiple time points from 0.5 to 48 h post dose by asking patients to rate how much the migraine was interfering with normal activities. Pooled data from SAMURAI and SPARTAN (SAMURAI + SPARTAN) and data from GLADIATOR were analyzed using the intention-to-treat populations.
Results: For SPARTAN + SAMURAI, significantly more patients who received lasmiditan at any dose versus placebo reported freedom from migraine-related functional disability at every timepoint from 2 h post dose, and this difference persisted to 48 h (p < 0.05). Significant differences from placebo in freedom from migraine-related functional disability commenced at 1 h post dose for lasmiditan 200 mg, 1.5 h for lasmiditan 100 mg, and 2 h for lasmiditan 50 mg. Findings from GLADIATOR supported those from SAMURAI + SPARTAN.
Conclusion: All doses of lasmiditan resulted in an improvement in migraine-related functional disability that persisted to 48 h. In SAMURAI + SPARTAN, a significant difference from placebo was observed as early as 1 h post dose. TRIAL REGISTRATION AT CLINICALTRIALS.GOV: SAMURAI (NCT02439320), SPARTAN (NCT02605174), and GLADIATOR (NCT02565186).
Keywords: Disability; Function; Lasmiditan; Migraine.
Figures


References
-
- Chaushev N, Milanov I. Impact of migraine and migraine treatment on patient’s capacity to work and quality of life. J Clin Med. 2009;2:26–31.
Associated data
LinkOut - more resources
Full Text Sources
Medical

