A cluster-randomized, non-inferiority trial comparing use of misoprostol for universal prophylaxis vs. secondary prevention of postpartum hemorrhage among community level births in Egypt
- PMID: 32448257
- PMCID: PMC7245883
- DOI: 10.1186/s12884-020-03008-5
A cluster-randomized, non-inferiority trial comparing use of misoprostol for universal prophylaxis vs. secondary prevention of postpartum hemorrhage among community level births in Egypt
Abstract
Background: Previous community-based research shows that secondary prevention of postpartum hemorrhage (PPH) with misoprostol only given to women with above-average measured blood loss produces similar clinical outcomes compared to routine administration of misoprostol for prevention of PPH. Given the difficulty of routinely measuring blood loss for all deliveries, more operational models of secondary prevention are needed.
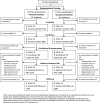
Methods: This cluster-randomized, non-inferiority trial included women giving birth with nurse-midwives at home or in Primary Health Units (PHUs) in rural Egypt. Two PPH management approaches were compared: 1) 600mcg oral misoprostol given to all women after delivery (i.e. primary prevention, current standard of care); 2) 800mcg sublingual misoprostol given only to women with 350-500 ml postpartum blood loss estimated using an underpad (i.e. secondary prevention). The primary outcome was mean change in pre- and post-delivery hemoglobin. Secondary outcomes included hemoglobin ≥2 g/dL and other PPH interventions.
Results: Misoprostol was administered after delivery to 100% (1555/1555) and 10.7% (117/1099) of women in primary and secondary prevention clusters, respectively. The mean drop in pre- to post-delivery hemoglobin was 0.37 (SD: 0.91) and 0.45 (SD: 0.76) among women in primary and secondary prevention clusters, respectively (difference adjusted for clustering = 0.01, one-sided 95% CI: < 0.27, p = 0.535). There were no statistically significant differences in secondary outcomes, including hemoglobin drop ≥2 g/dL, PPH diagnosis, transfer to higher level, or other interventions.
Conclusions: Misoprostol for secondary prevention of PPH is comparable to universal prophylaxis and can be implemented using local materials, such as underpads.
Trial registration: Clinicaltrials.gov NCT02226588, date of registration 27 August 2014.
Keywords: Home-births; Low and middle income countries; Midwives; Misoprostol; Postpartum hemorrhage; Prophylaxis; Secondary prevention; Task-sharing.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Testing a home-based model of care using misoprostol for prevention and treatment of postpartum hemorrhage: results from a randomized placebo-controlled trial conducted in Badakhshan province, Afghanistan.Reprod Health. 2020 Jun 5;17(1):88. doi: 10.1186/s12978-020-00933-8. Reprod Health. 2020. PMID: 32503556 Free PMC article. Clinical Trial.
-
Misoprostol for primary versus secondary prevention of postpartum haemorrhage: a cluster-randomised non-inferiority community trial.BJOG. 2016 Jan;123(1):120-7. doi: 10.1111/1471-0528.13540. Epub 2015 Sep 1. BJOG. 2016. PMID: 26333044 Free PMC article. Clinical Trial.
-
Sublingual misoprostol versus intramuscular oxytocin for prevention of postpartum hemorrhage in Uganda: a double-blind randomized non-inferiority trial.PLoS Med. 2014 Nov 4;11(11):e1001752. doi: 10.1371/journal.pmed.1001752. eCollection 2014 Nov. PLoS Med. 2014. PMID: 25369200 Free PMC article. Clinical Trial.
-
Misoprostol for postpartum hemorrhage prevention at home birth: an integrative review of global implementation experience to date.BMC Pregnancy Childbirth. 2013 Feb 20;13:44. doi: 10.1186/1471-2393-13-44. BMC Pregnancy Childbirth. 2013. PMID: 23421792 Free PMC article. Review.
-
Prevention of postpartum hemorrhage with misoprostol.Int J Gynaecol Obstet. 2007 Dec;99 Suppl 2:S198-201. doi: 10.1016/j.ijgo.2007.09.012. Epub 2007 Oct 24. Int J Gynaecol Obstet. 2007. PMID: 17961574 Review.
Cited by
-
Comparison of Clinical Efficacy and Safety between Misoprostol and Oxytocin in the Prevention of Postpartum Hemorrhage: A Meta-Analysis.J Healthc Eng. 2022 Apr 11;2022:3254586. doi: 10.1155/2022/3254586. eCollection 2022. J Healthc Eng. 2022. Retraction in: J Healthc Eng. 2023 Oct 4;2023:9895282. doi: 10.1155/2023/9895282. PMID: 35449871 Free PMC article. Retracted.
References
-
- Raghavan S, Geller S, Miller S, Goudar SS, Anger H, Yadavannavar MC, Dabash R, Bidri SR, Gudadinni MR, Udgiri R, et al. Misoprostol for primary versus secondary prevention of postpartum haemorrhage: a cluster-randomised non-inferiority community trial. BJOG. 2016;123(1):120–127. doi: 10.1111/1471-0528.13540. - DOI - PMC - PubMed
-
- Begley CM, Gyte GM, Devane D, McGuire W, Weeks A. Active versus expectant management for women in the third stage of labour. Cochrane Database Syst Rev. 2015;3:CD007412. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

