TFEB-NF-κB inflammatory signaling axis: a novel therapeutic pathway of Dihydrotanshinone I in doxorubicin-induced cardiotoxicity
- PMID: 32448281
- PMCID: PMC7245789
- DOI: 10.1186/s13046-020-01595-x
TFEB-NF-κB inflammatory signaling axis: a novel therapeutic pathway of Dihydrotanshinone I in doxorubicin-induced cardiotoxicity
Abstract
Background: Doxorubicin is effective in a variety of solid and hematological malignancies. Unfortunately, clinical application of doxorubicin is limited due to a cumulative dose-dependent cardiotoxicity. Dihydrotanshinone I (DHT) is a natural product from Salvia miltiorrhiza Bunge with multiple anti-tumor activity and anti-inflammation effects. However, its anti-doxorubicin-induced cardiotoxicity (DIC) effect, either in vivo or in vitro, has not been elucidated yet. This study aims to explore the anti-inflammation effects of DHT against DIC, and to elucidate the potential regulatory mechanism.
Methods: Effects of DHT on DIC were assessed in zebrafish, C57BL/6 mice and H9C2 cardiomyocytes. Echocardiography, histological examination, flow cytometry, immunochemistry and immunofluorescence were utilized to evaluate cardio-protective effects and anti-inflammation effects. mTOR agonist and lentivirus vector carrying GFP-TFEB were applied to explore the regulatory signaling pathway.
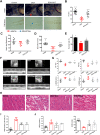
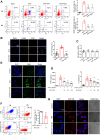
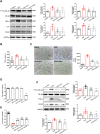
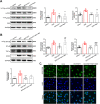
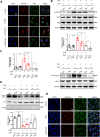
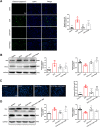
Results: DHT improved cardiac function via inhibiting the activation of M1 macrophages and the excessive release of pro-inflammatory cytokines both in vivo and in vitro. The activation and nuclear localization of NF-κB were suppressed by DHT, and the effect was abolished by mTOR agonist with concomitant reduced expression of nuclear TFEB. Furthermore, reduced expression of nuclear TFEB is accompanied by up-regulated phosphorylation of IKKα/β and NF-κB, while TFEB overexpression reversed these changes. Intriguingly, DHT could upregulate nuclear expression of TFEB and reduce expressions of p-IKKα/β and p-NF-κB.
Conclusions: Our results demonstrated that DHT can be applied as a novel cardioprotective compound in the anti-inflammation management of DIC via mTOR-TFEB-NF-κB signaling pathway. The current study implicates TFEB-IKK-NF-κB signaling axis as a previously undescribed, druggable pathway for DIC.
Keywords: Cardiotoxicity; Dihydrotanshinone I; Doxorubicin; Inflammation; TFEB-NF-κB.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Dehydroandrographolide ameliorates doxorubicin-mediated cardiotoxicity by regulating autophagy through the mTOR-TFEB pathway.Chem Biol Interact. 2024 Aug 25;399:111132. doi: 10.1016/j.cbi.2024.111132. Epub 2024 Jul 2. Chem Biol Interact. 2024. PMID: 38964637
-
Dihydrotanshinone exhibits an anti-inflammatory effect in vitro and in vivo through blocking TLR4 dimerization.Pharmacol Res. 2019 Apr;142:102-114. doi: 10.1016/j.phrs.2019.02.017. Epub 2019 Feb 19. Pharmacol Res. 2019. PMID: 30794925
-
Prx5 overexpression protect against doxorubicin-induced cardiotoxicity by inhibiting oxidative stress and inflammation via the TLR4/NF-κB pathway.Int Immunopharmacol. 2025 Jan 27;146:113788. doi: 10.1016/j.intimp.2024.113788. Epub 2024 Dec 19. Int Immunopharmacol. 2025. PMID: 39706046
-
Role of Oxidative Stress and Inflammation in Doxorubicin-Induced Cardiotoxicity: A Brief Account.Int J Mol Sci. 2024 Jul 8;25(13):7477. doi: 10.3390/ijms25137477. Int J Mol Sci. 2024. PMID: 39000584 Free PMC article. Review.
-
Iron metabolism in doxorubicin-induced cardiotoxicity: From mechanisms to therapies.Int J Biochem Cell Biol. 2024 Sep;174:106632. doi: 10.1016/j.biocel.2024.106632. Epub 2024 Jul 23. Int J Biochem Cell Biol. 2024. PMID: 39053765 Review.
Cited by
-
Quercetin Can Improve Spinal Cord Injury by Regulating the mTOR Signaling Pathway.Front Neurol. 2022 May 20;13:905640. doi: 10.3389/fneur.2022.905640. eCollection 2022. Front Neurol. 2022. PMID: 35669881 Free PMC article. Review.
-
Cardiovascular Toxicity of Antineoplastic Treatments in Hematological Diseases: Focus on Molecular Mechanisms to Improve Therapeutic Management.J Clin Med. 2024 Mar 9;13(6):1574. doi: 10.3390/jcm13061574. J Clin Med. 2024. PMID: 38541800 Free PMC article. Review.
-
Date Palm Pollen Extract Avert Doxorubicin-Induced Cardiomyopathy Fibrosis and Associated Oxidative/Nitrosative Stress, Inflammatory Cascade, and Apoptosis-Targeting Bax/Bcl-2 and Caspase-3 Signaling Pathways.Animals (Basel). 2021 Mar 20;11(3):886. doi: 10.3390/ani11030886. Animals (Basel). 2021. PMID: 33804672 Free PMC article.
-
Cardiotoxicity of Zebrafish Induced by 6-Benzylaminopurine Exposure and Its Mechanism.Int J Mol Sci. 2022 Jul 29;23(15):8438. doi: 10.3390/ijms23158438. Int J Mol Sci. 2022. PMID: 35955574 Free PMC article.
-
A universal gene expression signature-based strategy for the high-throughput discovery of anti-inflammatory drugs.Inflamm Res. 2025 Jan 7;74(1):2. doi: 10.1007/s00011-024-01968-4. Inflamm Res. 2025. PMID: 39762416 Free PMC article.
References
-
- Damiani RM, Moura DJ, Viau CM, Caceres RA, Henriques JAP, Saffi J. Pathways of cardiac toxicity: comparison between chemotherapeutic drugs doxorubicin and mitoxantrone. Arch Toxicol. 2016;90(9):2063–2076. - PubMed
-
- Briasoulis A, Androulakis E, Christophides T, Tousoulis D. The role of inflammation and cell death in the pathogenesis, progression and treatment of heart failure. Heart Fail Rev. 2016;21(2):169–176. - PubMed
-
- Akolkar G, da Silva DD, Ayyappan P, Bagchi AK, Jassal DS, Salemi VMC, Irigoyen MC, De Angelis K, Singal PK. Vitamin C mitigates oxidative/nitrosative stress and inflammation in doxorubicin-induced cardiomyopathy. Am J Physiol Heart Circ Physiol. 2017;313(4):H795–H809. - PubMed
MeSH terms
Substances
Grants and funding
- 81822049/National Natural Science Foundation of China
- 81673712/National Natural Science Foundation of China
- 81673802/National Natural Science Foundation of China
- 2019ZX09201004-001-011/National Key New Drug Creation and Manufacturing Program, Ministry of Science and Technology
- 151044/Fok Ying Tung Education Foundation
LinkOut - more resources
Full Text Sources
Miscellaneous

