Dihydromyricetin attenuates Escherichia coli lipopolysaccharide-induced ileum injury in chickens by inhibiting NLRP3 inflammasome and TLR4/NF-κB signalling pathway
- PMID: 32448367
- PMCID: PMC7247275
- DOI: 10.1186/s13567-020-00796-8
Dihydromyricetin attenuates Escherichia coli lipopolysaccharide-induced ileum injury in chickens by inhibiting NLRP3 inflammasome and TLR4/NF-κB signalling pathway
Abstract
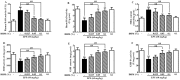
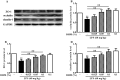
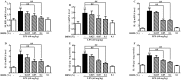
Lipopolysaccharide (LPS) as a major component of Escherichia coli cell wall can cause inflammation and cell death. Dihydromyricetin (ampelopsin, DHM) is a natural flavonoid compound with anti-inflammatory, anti-oxidant and anti-bacterial effects. The preventive effects of DHM against ileum injury remain unclear. Here, we explored the protective role of DHM against LPS-induced ileum injury in chickens. In this study, DHM significantly attenuated LPS-induced alteration in diamine oxidase, malondialdehyde, reduced glutathione, glutathione peroxidase and superoxide dismutase levels in chicken plasma and ileum. Histology evaluation showed that the structure of blood vessels in ileum was seriously fragmented and presence of necrotic tissue in the lumen in the LPS group. Scanning electron microscopic observation revealed that the surface of the villi was rough and uneven, the structure was chaotic, and the normal finger shape was lost in the LPS group. In contrast, 0.05% and 0.1% DHM treatment partially alleviated the abnormal morphology. Additionally, DHM maintained the barrier function by restoring the protein expression of occludin, claudin-1 and zonula occludens protein-1. DHM inhibited apoptosis through the reduction of the expression of bax and caspase-3 and restored the expression of bcl-2. Importantly, DHM could reduce ileum NLR family pyrin domain-containing 3 (NLRP3), caspase-1, interleukin (IL)-1β and IL-18 expression to protect tissues from pyroptosis and inhibited toll-like receptor 4 (TLR4)/nuclear factor kappa-B (NF-κB) signalling pathway. In summary, DHM attenuated the ileum mucosal damage, oxidative stress and apoptosis, maintained barrier function, inhibited NLRP3 inflammasome and TLR4/NF-κB signalling pathway activation triggered by Escherichia coli LPS.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- Ghunaim H, Abu-Madi MA, Kariyawasam S. Advances in vaccination against avian pathogenic Escherichia coli respiratory disease: potentials and limitations. Vet Microbiol. 2014;172:13–22. - PubMed
-
- Zhang Q, Chen X, Eicher S, Ajuwon K, Applegate T. Effect of threonine on secretory immune system using a chicken intestinal ex vivo model with lipopolysaccharide challenge. Poult Sci. 2017;96:3043–3051. - PubMed
-
- Li R, Li J, Zhang S, Mi Y, Zhang C. Attenuating effect of melatonin on lipopolysaccharide-induced chicken small intestine inflammation. Poult Sci. 2018;97:2295–2302. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

