Risk factors for genital infections in people initiating SGLT2 inhibitors and their impact on discontinuation
- PMID: 32448787
- PMCID: PMC7252998
- DOI: 10.1136/bmjdrc-2020-001238
Risk factors for genital infections in people initiating SGLT2 inhibitors and their impact on discontinuation
Abstract
Introduction: To identify risk factors, absolute risk, and impact on treatment discontinuation of genital infections with sodium-glucose co-transporter-2 inhibitors (SGLT2i).
Research design and methods: We assessed the relationship between baseline characteristics and genital infection in 21 004 people with type 2 diabetes initiating SGLT2i and 55 471 controls initiating dipeptidyl peptidase-4 inhibitors (DPP4i) in a UK primary care database. We assessed absolute risk of infection in those with key risk factors and the association between early genital infection and treatment discontinuation.
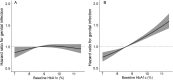
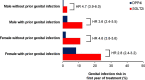
Results: Genital infection was substantially more common in those treated with SGLT2i (8.1% within 1 year) than DPP4i (1.8%). Key predictors of infection with SGLT2i were female sex (HR 3.64; 95% CI 3.23 to 4.11) and history of genital infection; <1 year before initiation (HR 4.38; 3.73 to 5.13), 1-5 years (HR 3.04; 2.64 to 3.51), and >5 years (HR 1.79; 1.55 to 2.07). Baseline HbA1c was not associated with infection risk for SGLT2i, in contrast to DPP4i where risk increased with higher HbA1c. One-year absolute risk of genital infection with SGLT2i was highest for those with a history of prior infection (females 23.7%, males 12.1%), compared with those without (females 10.8%, males 2.7%). Early genital infection was associated with a similar discontinuation risk for SGLT2i (HR 1.48; 1.21-1.80) and DPP4i (HR 1.58; 1.21-2.07).
Conclusions: Female sex and history of prior infection are simple features that can identify subgroups at greatly increased risk of genital infections with SGLT2i therapy. These data can be used to risk-stratify patients. High HbA1c is not a risk factor for genital infections with SGLT2i.
Keywords: A1C; adherence to medications; candida; non-insulin treated type 2 diabetes.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: APM declares research support from Eli Lilly and Company, Pfizer, and AstraZeneca. NAS has consulted or been on speakers bureaus for Amgen, Astrazeneca, Boehringer Ingelheim, Janssen, Eli-Lilly, Novo Nordisk, NAPP pharmaceuticals, Pfizer, and Sanofi and received grant support from Boehringer Ingelheim. ERP declares personal fees from Eli Lilly and Company, Novo Nordisk, and AstraZeneca. RRH reports research support from AstraZeneca, Bayer and Merck Sharp & Dohme, and personal fees from Bayer, Intarcia, Merck Sharp & Dohme, Novartis, and Novo Nordisk. AGJ is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Figures

References
-
- Authors/Task Force Members, Rydén L, Grant PJ, et al. . Esc guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the task force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of cardiology (ESC) and developed in collaboration with the European association for the study of diabetes (EASD). Eur Heart J 2013;34:3035–87. 10.1093/eurheartj/eht108 - DOI - PubMed
-
- National Institute for Health and Care Excellence NICE guideline [NG28] - Type 2 diabetes in adults: management, 2019. Available: https://www.nice.org.uk/guidance/ng28 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
