Multicentric study of cervical cancer screening with human papillomavirus testing and assessment of triage methods in Latin America: the ESTAMPA screening study protocol
- PMID: 32448795
- PMCID: PMC7252979
- DOI: 10.1136/bmjopen-2019-035796
Multicentric study of cervical cancer screening with human papillomavirus testing and assessment of triage methods in Latin America: the ESTAMPA screening study protocol
Abstract
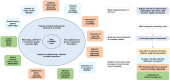
Introduction: Human papillomavirus (HPV) testing is replacing cytology in primary screening. Its limited specificity demands using a second (triage) test to better identify women at high-risk of cervical disease. Cytology represents the immediate triage but its low sensitivity might hamper HPV testing sensitivity, particularly in low-income and middle-income countries (LMICs), where cytology performance has been suboptimal. The ESTAMPA (EStudio multicéntrico de TAMizaje y triaje de cáncer de cuello uterino con pruebas del virus del PApiloma humano; Spanish acronym) study will: (1) evaluate the performance of different triage techniques to detect cervical precancer and (2) inform on how to implement HPV-based screening programmes in LMIC.
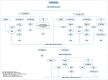
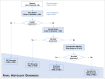
Methods and analysis: Women aged 30-64 years are screened with HPV testing and Pap across 12 study centres in Latin America. Screened positives have colposcopy with biopsy and treatment of lesions. Women with no evident disease are recalled 18 months later for another HPV test; those HPV-positive undergo colposcopy with biopsy and treatment as needed. Biological specimens are collected in different visits for triage testing, which is not used for clinical management. The study outcome is histological high-grade squamous intraepithelial or worse lesions (HSIL+) under the lower anogenital squamous terminology. About 50 000 women will be screened and 500 HSIL+ cases detected (at initial and 18 months screening). Performance measures (sensitivity, specificity and predictive values) of triage techniques to detect HSIL+ will be estimated and compared with adjustment by age and study centre.
Ethics and dissemination: The study protocol has been approved by the Ethics Committee of the International Agency for Research on Cancer (IARC), of the Pan American Health Organisation (PAHO) and by those in each participating centre. A Data and Safety Monitoring Board (DSMB) has been established to monitor progress of the study, assure participant safety, advice on scientific conduct and analysis and suggest protocol improvements. Study findings will be published in peer-reviewed journals and presented at scientific meetings.
Trial registration number: NCT01881659.
Keywords: colposcopy; gynaecological oncology; molecular diagnostics; preventive medicine; public health; risk management.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures


References
-
- Ferlay J, Ervik M, Lam F, et al. . Global cancer Observatory: cancer today Lyon, France: international agency for research on cancer, 2018. Available: http://gco.iarc.fr/today; [Accessed 01 Jul 2019].
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
