Pharmacokinetics and Safety of a Raltegravir-Containing Regimen in Children Aged 4 Weeks to 2 Years Living With Human Immunodeficiency Virus and Receiving Rifampin for Tuberculosis
- PMID: 32448902
- PMCID: PMC7996637
- DOI: 10.1093/jpids/piaa039
Pharmacokinetics and Safety of a Raltegravir-Containing Regimen in Children Aged 4 Weeks to 2 Years Living With Human Immunodeficiency Virus and Receiving Rifampin for Tuberculosis
Abstract
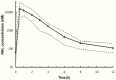
Pharmacological interactions limit treatment options for children living with human immunodeficiency virus (HIV) and tuberculosis (TB). We found that 12 mg/kg twice daily raltegravir chewable tablets (administered after crushing) safely achieved pharmacokinetic targets in children living with HIV aged 4 weeks to <2 years receiving concurrent rifampin to treat TB.
Clinical trials registration: NCT01751568.
Keywords: antiretroviral therapy; drug interactions; pediatrics; rifampin; tuberculosis.
© The Author(s) 2020. Published by Oxford University Press on behalf of The Journal of the Pediatric Infectious Diseases Society. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
References
-
- World Health Organization. Global tuberculosis report 2018. World Health Organization. 2018. Available at: https://apps.who.int/iris/handle/10665/274453. Accessed November 20, 2019.
-
- World Health Organization. Guidelines for treatment of drug-susceptible tuberculosis and patient care, 2017 update. 2017. Available at: https://apps.who.int/iris/bitstream/handle/10665/255052/9789241550000-en.... Accessed November 20, 2019.
-
- Ren Y, Nuttall JJ, Eley BS, et al. Effect of rifampicin on efavirenz pharmacokinetics in HIV-infected children with tuberculosis. J Acquir Immune Defic Syndr 2009; 50:439–43. - PubMed