Non-nutritive sweeteners for diabetes mellitus
- PMID: 32449201
- PMCID: PMC7387865
- DOI: 10.1002/14651858.CD012885.pub2
Non-nutritive sweeteners for diabetes mellitus
Abstract
Background: Products sweetened with non-nutritive sweeteners (NNS) are widely available. Many people with type 1 or type 2 diabetes use NNS as a replacement for nutritive sweeteners to control their carbohydrate and energy intake. Health outcomes associated with NNS use in diabetes are unknown.
Objectives: To assess the effects of non-nutritive sweeteners in people with diabetes mellitus.
Search methods: We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE Ovid, Scopus, the WHO ICTRP, and ClinicalTrials.gov. The date of the last search of all databases (except for Scopus) was May 2019. We last searched Scopus in January 2019. We did not apply any language restrictions.
Selection criteria: We included randomised controlled trials (RCTs) with a duration of four weeks or more comparing any type of NNS with usual diet, no intervention, placebo, water, a different NNS, or a nutritive sweetener in individuals with type 1 or type 2 diabetes. Trials with concomitant behaviour-changing interventions, such as diet, exercise, or both, were eligible for inclusion, given that the concomitant interventions were the same in the intervention and comparator groups.
Data collection and analysis: Two review authors independently screened abstracts, full texts, and records retrieved from trials registries, assessed the certainty of the evidence, and extracted data. We used a random-effects model to perform meta-analysis, and calculated effect estimates as risk ratios (RRs) for dichotomous outcomes and mean differences (MDs) for continuous outcomes, using 95% confidence intervals (CIs). We assessed risk of bias using the Cochrane 'Risk of bias' tool and the certainty of evidence using the GRADE approach.
Main results: We included nine RCTs that randomised a total of 979 people with type 1 or type 2 diabetes. The intervention duration ranged from 4 to 10 months. We judged none of these trials as at low risk of bias for all 'Risk of bias' domains; most of the included trials did not report the method of randomisation. Three trials compared the effects of a dietary supplement containing NNS with sugar: glycosylated haemoglobin A1c (HbA1c) was 0.4% higher in the NNS group (95% CI -0.5 to 1.2; P = 0.44; 3 trials; 72 participants; very low-certainty evidence). The MD in weight change was -0.1 kg (95% CI -2.7 to 2.6; P = 0.96; 3 trials; 72 participants; very low-certainty evidence). None of the trials with sugar as comparator reported on adverse events. Five trials compared NNS with placebo. The MD for HbA1c was 0%, 95% CI -0.1 to 0.1; P = 0.99; 4 trials; 360 participants; very low-certainty evidence. The 95% prediction interval ranged between -0.3% and 0.3%. The comparison of NNS versus placebo showed a MD in body weight of -0.2 kg, 95% CI -1 to 0.6; P = 0.64; 2 trials; 184 participants; very low-certainty evidence. Three trials reported the numbers of participants experiencing at least one non-serious adverse event: 36/113 participants (31.9%) in the NNS group versus 42/118 participants (35.6%) in the placebo group (RR 0.78, 95% CI 0.39 to 1.56; P = 0.48; 3 trials; 231 participants; very low-certainty evidence). One trial compared NNS with a nutritive low-calorie sweetener (tagatose). HbA1c was 0.3% higher in the NNS group (95% CI 0.1 to 0.4; P = 0.01; 1 trial; 354 participants; very low-certainty evidence). This trial did not report body weight data and adverse events. The included trials did not report data on health-related quality of life, diabetes complications, all-cause mortality, or socioeconomic effects.
Authors' conclusions: There is inconclusive evidence of very low certainty regarding the effects of NNS consumption compared with either sugar, placebo, or nutritive low-calorie sweetener consumption on clinically relevant benefit or harm for HbA1c, body weight, and adverse events in people with type 1 or type 2 diabetes. Data on health-related quality of life, diabetes complications, all-cause mortality, and socioeconomic effects are lacking.
Trial registration: ClinicalTrials.gov NCT00955747 NCT01324921 NCT02252952 NCT02412774 NCT02487537 NCT02813759 NCT03680482.
Copyright © 2020 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
SL: was financially supported by the Alexander von Humboldt Foundation.
DK: none known.
IT: none known.
TF: none known.
JM: we have received financial support from the World Health Organization (WHO) to conduct a systematic review on health effects of non‐sugar sweeteners in healthy adults and children.
HS: none known.
Figures

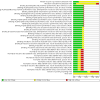
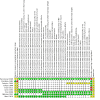



































Comment in
-
Effects of non-nutritive sweeteners on diabetes: Comments on a Cochrane review.Diabet Med. 2021 Sep;38(9):e14520. doi: 10.1111/dme.14520. Epub 2021 Jan 27. Diabet Med. 2021. PMID: 33428784 No abstract available.
-
Effects of non-nutritive sweeteners on diabetes: Reply to Laviada-Molina et al.Diabet Med. 2021 Sep;38(9):e14589. doi: 10.1111/dme.14589. Epub 2021 May 10. Diabet Med. 2021. PMID: 33932307 No abstract available.
References
References to studies included in this review
Barriocanal 2008 {published data only}
-
- Barriocanal LA, Palacios M, Benitez G, Benitez S, Jimenez JT, Jimenez N, et al. Apparent lack of pharmacological effect of steviol glycosides used as sweeteners in humans. A pilot study of repeated exposures in some normotensive and hypotensive individuals and in type 1 and type 2 diabetics. Regulatory Toxicology and Pharmacology : RTP 2008;51(1):37-41. - PubMed
Chantelau 1985 {published data only}
-
- Chantelau EA, Gosseringer G, Sonnenberg GE, Berger M. Moderate intake of sucrose does not impair metabolic control in pump-treated diabetic out-patients. Diabetologia 1985;28(4):204-7. - PubMed
Colagiuri 1989 {published data only}
-
- Colagiuri S, Miller JJ, Edwards RA. Metabolic effects of adding sucrose and aspartame to the diet of subjects with noninsulin-dependent diabetes mellitus. American Journal of Clinical Nutrition 1989;50(3):474-8. - PubMed
Cooper 1988 {published data only}
-
- Cooper PL, Wahlqvist ML, Simpson RW. Sucrose versus saccharin as an added sweetener in non-insulin-dependent diabetes: short- and medium-term metabolic effects. Diabetic Medicine 1988;5(7):676-80. - PubMed
Ensor 2015 {published data only}
-
- CTRI/2009/091/000536. Effects of Naturlose (Tagatose) on blood sugar control and safety of Naturlose over one year in subjects with type 2 diabetes under diet control and exercise. apps.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2009/091/000536 (first received 17 April 2007; last updated 21 May 2019).
-
- NCT00955747. Naturlose (D-Tagatose) efficacy evaluation trial (NEET). clinicaltrials.gov/ct2/show/study/NCT00955747 (first received 10 August 2009; last updated 15 November 2014).
Grotz 2003 {published data only}
-
- Grotz VL, Henry RR, McGill JB, Prince MJ, Shamoon H, Trout JR, et al. Lack of effect of sucralose on glucose homeostasis in subjects with type 2 diabetes. Journal of the American Dietetic Association 2003;103(12):1607-12. - PubMed
Maki 2008 {published data only}
-
- Maki KC, Curry LL, Reeves MS, Toth PD, McKenney JM, Farmer MV, et al. Chronic consumption of rebaudioside A, a steviol glycoside, in men and women with type 2 diabetes mellitus. Food and Chemical Toxicology 2008;46 Suppl 7:47-53. - PubMed
Nehrling 1985 {published data only}
-
- Nehrling JK, Kobe P, McLane MP, Olson RE, Kamath S, Horwitz DL. Aspartame use by persons with diabetes. Diabetes Care 1985;8(5):415-7. - PubMed
Stern 1976 {published data only}
-
- Stern SB, Bleicher SJ, Flores A, Gombos G, Recitas D, Shu J. Administration of aspartame in non-insulin-dependent diabetics. Journal of Toxicology & Environmental Health 1976;2(2):429-39. - PubMed
References to studies excluded from this review
ACTRN12618000862246 {published data only}
-
- ACTRN12618000862246. Do low-calorie sweeteners influence intestinal glucose absorption in patients with type 2 diabetes? www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=375119 (first received 22 May 2018).
Anonymous 1979 {published data only}
-
- No authors listed. Saccharin substitutes for diabetics. Geriatrics 1979;34(10):15. - PubMed
Barbosa Martín 2014 {published data only}
-
- Barbosa-Martín E, Sabido-Cortés D, Aranda-Gozález I, Betancur-Ancona D. Use of stevia rebaudiana extract as a sweetener of chocolates for people with diabetes. In: Stevia rebaudiana: Chemical Composition, Uses and Health Promoting Aspects. Nova Science Publishers, 2014:147-58.
Bastaki 2015 {published data only}
-
- Bastaki S. Pharmacotherapy of nonnutritive sweeteners in diabetes mellitus. International Journal of Diabetes and Metabolism 2015;23(1):11-2.
Baturina 2004 {published data only}
-
- Baturina BA, Sharafetdinov XX, Meshcheriakova BA, Plotnikova OA, Sokolov AI, Gapparov MM. Effect of food additive Neotame (N-[N-(3,3-dimethylbutyl)-L-alpha-aspartyl]-L-phenylalanine-1-methyl) on glucose level in blood of patients with diabetes mellitus type 2. Voprosy Pitaniia 2004;73(6):18-20. - PubMed
Beringer 1973 {published data only}
-
- Beringer A. Are sweetening substances dangerous for diabetics? Wiener Medizinische Wochenschrift 1973;123(4):41-8. - PubMed
Blackburn 1997 {published data only}
-
- Blackburn GL, Kanders BS, Lavin PT, Keller SD, Whatley J. The effect of aspartame as part of a multidisciplinary weight-control program on short- and long-term control of body weight. American Journal of Clinical Nutrition 1997;65(2):409-18. - PubMed
Bloomgarden 2011 {published data only}
Chantelau 1986 {published data only}
-
- Chantelau E. Sugar substitutes in the diet therapy of type I diabetes mellitus. Deutsche Medizinische Wochenschrift 1986;111(31-32):1220-2. - PubMed
Corfe 1858 {published data only}
-
- Corfe G. Diabetes treated by saccharine food. BMJ 1858;s4-1(58):102-3.
Deschamps 1971 {published data only}
-
- Deschamps I, Tichet J, Lestradet H. Influence of cyclamate on blood sugar in normal and diabetic children. Diabete 1971;19(1):21-3. - PubMed
Dinkovski 2017 {published data only}
-
- Dinkovski N. Natural sweetener is suitable for diabetics. www.foodmanufacture.co.uk/Article/2017/05/01/Natural-sweetener-is-suitab... (accessed 25 January 2018).
EUCTR2006‐002395‐18‐DK {published data only}
-
- EUCTR2006-002395-18-DK. Intervention studier med steviol til belysning af dosis respons forhold samt langtidseffekt hos personer med type 2 diabetes. www.clinicaltrialsregister.eu/ctr-search/trial/2006-002395-18/DK (accessed 3 August 2018).
Farkas 1965 {published data only}
-
- Farkas CS, Forbes CE. Do non-caloric sweeteners aid patients with diabetes to adhere to their diets? Journal of the American Dietetic Association 1965;46:482-4. - PubMed
Ferland 2007 {published data only}
-
- Ferland A, Brassard P, Poirier P. Is aspartame really safer in reducing the risk of hypoglycemia during exercise in patients with type 2 diabetes? Diabetes Care 2007;30(7):e59. - PubMed
Ferri 2006 {published data only}
-
- Ferri LA, Alves-Do-Prado W, Yamada SS, Gazola S, Batista MR, Bazotte RB. Investigation of the antihypertensive effect of oral crude stevioside in patients with mild essential hypertension. Phytotherapy Research 2006;20(9):732-6. - PubMed
Fukuda 2010 {published data only}
-
- Fukuda M, Terata T, Tsuda K, Sugawara M, Kitatani N, Seino Y. Aspartame-acesulfame K-containing low-energy erythritol sweetener markedly suppresses postprandial hyperglycemia in mild and borderline diabetics. Food Science and Technology Research 2010;16(5):457-66.
Gapparov 1996 {published data only}
-
- Gapparov MM. Sugar substitutes in specialized child nutrition products for the prevention and treatment of diabetes mellitus. Voprosy Pitaniia 1996;5:68-70. - PubMed
Healy 2013 {published data only}
-
- Healy AM. Artificial sweeteners and high-fructose corn syrup: effects on diabetes and weight. Integrative Medicine Alert 2013;16(10):114-9.
Heraud 1976 {published data only}
-
- Heraud G, Roux E. Chemical sweeteners in current dietetics applied to diabetic patients. Ouest Medical 1976;29(7):503-6.
IRCT2015091513612N6 {published data only}IRCT2015091513612N6
-
- IRCT2015091513612N6. Comparison of glycemic control in patients with type 2 diabetes on regular diabetic diet or artificial sweeteners. apps.who.int/trialsearch/Trial3.aspx?trialid=IRCT2015091513612N6 (accessed 14 January 2019).
Kanders 1988 {published data only}
-
- Kanders BS, Lavin PT, Kowalchuk MB, Greenberg I, Blackburn GL. An evaluation of the effect of aspartame on weight loss. Appetite 1988;11 Suppl 1:73-84. - PubMed
Knopp 1976 {published data only}
-
- Knopp RH, Brandt K, Arky RA. Effects of aspartame in young persons during weight reduction. Journal of Toxicology and Environmental Health 1976;2(2):417-28. - PubMed
Leon 1989 {published data only}
-
- Leon AS, Hunninghake DB, Bell C, Rassin DK, Tephly TR. Safety of long-term large doses of aspartame. Archives of Internal Medicine 1989;149(10):2318-24. - PubMed
Macdonald 1970 {published data only}
-
- Macdonald I. The therapeutic potential of artificial sweeteners. Practitioner 1970;204(220):268-70. - PubMed
Madjd 2017 {published data only}
-
- Madjd A, Taylor MA, Delavari A, Malekzadeh R, Macdonald IA, Farshchi HR. Beneficial effects of replacing diet beverages with water on type 2 diabetic obese women following a hypo-energetic diet: a randomized, 24-week clinical trial. Diabetes, Obesity & Metabolism 2017;19(1):125-32. - PubMed
Maersk 2012 {published data only}
-
- Maersk M, Belza A, Stødkilde-Jørgensen H, Ringgaard S, Chabanova E, Thomsen H, et al. Sucrose-sweetened beverages increase fat storage in the liver, muscle, and visceral fat depot: a 6-mo randomized intervention study. American Journal of Clinical Nutrition 2012;95(2):283-9. - PubMed
Maki 2009 {published data only}
-
- Maki KC, Curry LL, McKenney JM, Farmer MV, Reeves MS, Dicklin MR, et al. Glycemic and blood pressure responses to acute doses of rebaudioside A, a steviol glycoside, in subjects with normal glucose tolerance or type 2 diabetes mellitus. FASEB Journal. Conference: Experimental Biology 2009;23(1 Suppl):351.6.
Masic 2017 {published data only}
-
- Masic U, Harrold JA, Christiansen P, Cuthbertson DJ, Hardman CA, Robinson E, et al. Effects of non-nutritive sweetened beverages on appetite during active weight loss (SWITCH): protocol for a randomized, controlled trial assessing the effects of non-nutritive sweetened beverages compared to water during a 12-week weight loss period and a follow up weight maintenance period. Contemporary Clinical Trials 2017;53:80-8. - PubMed
Mazovetskii 1976 {published data only}
-
- Mazovetskii AG. Sugar substitutes in the treatment of diabetes mellitus. Sovetskaia Meditsina 1976;6:93-6. - PubMed
McCann 1956 {published data only}
-
- McCann MB, Trulson MF, Stulb SC. Non-caloric sweeteners and weight reduction. Journal of the American Dietetic Association 1956;32(4):327-30. - PubMed
Mehnert 1975 {published data only}
-
- Mehnert H, Dietze G, Haslbeck M. Sugar and sugar substitutes in dietary treatment of disorders of carbohydrate metabolism. Nutrition and Metabolism 1975;18(Suppl 1):171-90. - PubMed
Mehnert 1979 {published data only}
-
- Mehnert H, Foerster H. Oral administration of fructose as sugar substitute in the diet of diabetes mellitus patients. Aktuelle Ernahrungsmedizin Klinik und Praxis 1979;4(4):178-93.
Morris 1993 {published data only}
-
- Morris DH, Cuneo P, Stuart MJ, Mance MJ, Bell KJ, Puleo E, et al. High-intensity sweetener, energy and nutrient intakes of overweight women and men participating in a weight-loss program. Nutrition Research (New York, NY) 1993;13(2):123-32.
NCT01324921 {published data only}
-
- NCT01324921. Effect of nutritional products on metabolic parameters in subjects with type 2 diabetics. clinicaltrials.gov/show/NCT01324921 (first received 29 March 2011).
NCT02252952 {published data only}
-
- NCT02252952. The effect of sugar sweetened and diet beverages consumed as part of a weight-maintenance diet on fat storage. clinicaltrials.gov/ct2/show/NCT02252952 (first received 30 September 2014).
NCT02412774 {published data only}
-
- NCT02412774. Effects of replacing diet beverages with water on weight loss and plasma glucose control in type 2 diabetes. clinicaltrials.gov/ct2/show/NCT02412774 (first received 9 April 2015).
NCT02487537 {published data only}
-
- NCT02487537. Immediate and long-term induction of incretin release by artificial sweeteners 2 (ILIAS-2). clinicaltrials.gov/show/NCT02487537 (first received 1 July 2015).
NCT02813759 {published data only}
-
- NCT02813759. Sucralose in subjects with diabetes mellitus insulin requesting (SDMIR). clinicaltrials.gov/ct2/show/record/NCT02813759 (first received 27 June 2016).
NCT03680482 {published data only}
-
- NCT03680482. To compare the effects of non-nutritive sweeteners intake in subjects with T2DM. clinicaltrials.gov/ct2/show/NCT03680482 (first received 21 September 2018).
Noren 2014 {published data only}
Odegaard 2017 {published data only}
-
- Odegaard A, Hirahatake K. The effect of diet beverage intake on measures of diabetes control: a pilot study. Circulation 2017 March 7;135(Suppl 1):AP293.
PACTR201410000894447 {published data only}
-
- PACTR201410000894447. Efficacy and cost of stevia rebaudiana bertoni extract as adjunctive therapy in Sahrawi patients with type 2 diabetes. pactr.samrc.ac.za/Search.aspx (accessed 27 January 2018).
Parimalavalli 2011 {published data only}
-
- Parimalavalli R, Radhaisri S. Glycaemic index of stevia product and its efficacy on blood glucose level in type 2 diabetes. Indian Journal of Science and Technology 2011;4(3):318-21.
Peters 2014 {published data only}
-
- Peters JC, Wyatt HR, Foster GD, Pan Z, Wojtanowski AC, Vander Veur SS, et al. The effects of water and non-nutritive sweetened beverages on weight loss during a 12-week weight loss treatment program. Obesity (Silver Spring, MD) 2014;22(6):1415-21. - PubMed
Peters 2016 {published data only}
Piernas 2011 {published data only}
-
- Piernas C, Tate DF, Popkin BM. Does diet beverage intake affect consumption patterns? Results from the choice RCT study. Obesity (Silver Spring, MD) 2011;19:S70.
Piernas 2013 {published data only}
Prols 1973 {published data only}
-
- Prols H, Haslbeck M, Mehnert H. Investigations into the action of high doses of saccharin on the metabolism in diabetics. Deutsche Medizinische Wochenschrift 1973;98(41):1901-4. - PubMed
Pröls 1974 {published data only}
-
- Pröls H, Wittmann P, Haslbeck M, Mehnert H. Investigations on the effect of high sodium cyclamate doses on the metabolism of diabetics. Munchener Medizinische Wochenschrift (1950) 1974;116(43):1885-8.
Purdy 1988 {published data only}
-
- Purdy CW. The use of saccharin in diabetes. JAMA 1988;259(8):1260.
Reid 1994 {published data only}
-
- Reid M, Hammersley R. The effects of sucrose on everyday eating in normal weight men and women. Appetite 1994;22(3):221-31. - PubMed
Reid 1998 {published data only}
-
- Reid M, Hammersley R. The effects of sugar on subsequent eating and mood in obese and non-obese women. Psychology, Health & Medicine 1998;3(3):299-313.
Reid 2010 {published data only}
-
- Reid M, Hammersley R, Duffy M. Effects of sucrose drinks on macronutrient intake, body weight, and mood state in overweight women over 4 weeks. Appetite 2010;55(1):130-6. - PubMed
Reyna 2003 {published data only}
-
- Reyna NY, Cano C, Bermudez VJ, Medina MT, Souki AJ, Ambard M, et al. Sweeteners and beta-glucans improve metabolic and anthropometrics variables in well controlled type 2 diabetic patients. American Journal of Therapeutics 2003;10(6):438-43. - PubMed
Ritu 2016 {published data only}
-
- Ritu M, Nandini J. Nutritional composition of Stevia rebaudiana, a sweet herb, and its hypoglycaemic and hypolipidaemic effect on patients with non-insulin dependent diabetes mellitus. Journal of the Science of Food & Agriculture 2016;96(12):4231-4. - PubMed
Rodin 1990 {published data only}
-
- Rodin J. Comparative effects of fructose, aspartame, glucose, and water preloads on calorie and macronutrient intake. American Journal of Clinical Nutrition 1990;51(3):428-35. - PubMed
Rogers 1994 {published data only}
-
- Rogers PJ, Blundell JE. Reanalysis of the effects of phenylalanine, alanine, and aspartame on food intake in human subjects. Physiology & Behavior 1994;56(2):247-50. - PubMed
Sadeghi 2019 {published data only}
Samanta 1985 {published data only}
-
- Samanta A, Burden AC, Jones GR. Plasma glucose responses to glucose, sucrose, and honey in patients with diabetes mellitus: an analysis of glycaemic and peak incremental indices. Diabetic Medicine 1985;2(5):371-3. - PubMed
Saundby 1887 {published data only}
-
- Saundby R. Jambul in diabetes; saccharine in diabetes. Lancet 1887;130(3347):834.
Schatz 1977 {published data only}
-
- Schatz H, Winkler G, Pfeiffer EF. Sweeteners and sugar exchange foods in the diet of juvenile diabetics. Munchener Medizinische Wochenschrift 1977;119(7):213-4. - PubMed
Sharafetdinov 2002 {published data only}
-
- Sharafetdinov KK, Meshcheriakova VA, Plotnikova OA, Gapparov MG. Comparative study of postprandial glycaemia in type 2 diabetic patients after consumption of mono- and disaccharides and sweeteners. Voprosy Pitaniia 2002;71(2):22-6. - PubMed
Shigeta 1985 {published data only}
-
- Shigeta H, Yoshida T, Nakai M, Mori H, Kano Y, Nishioka H, et al. Effects of aspartame on diabetic rats and diabetic patients. Journal of Nutritional Science and Vitaminology 1985;31(5):533-40. - PubMed
Simeonov 2002 {published data only}
-
- Simeonov SB, Botushanov NP, Karahanian EB, Pavlova MB, Husianitis HK, Troev DM. Effects of Aronia melanocarpa juice as part of the dietary regimen in patients with diabetes mellitus. Folia Medica (Plovdiv) 2002;44(3):20-3. - PubMed
Skyler 1980 {published data only}
-
- Skyler JS, Miller NE. The use of sweeteners by diabetic patients. Practical Cardiology 1980;6(10):119-29.
Sloane 1858 {published data only}
-
- Sloane J. Leicester infirmary: observations on the saccharine treatment of diabetes mellitus. British Medical Journal 1858;s4-1(74):425-7.
Stevens 2013 {published data only}
-
- Stevens HC. Diabetes and diet beverage study has serious limitations. American Journal of Clinical Nutrition 2013;98(1):248-9. - PubMed
Stoye 2008 {published data only}
-
- Stoye U, Schmutz E, Krebs S, Koch S. Expert advice: Stevia. Zeitschrift fur Phytotherapie 2008;29(3):137.
Sørensen 2014 {published data only}
-
- Sørensen LB, Vasilaras TH, Astrup A, Raben A. Sucrose compared with artificial sweeteners: a clinical intervention study of effects on energy intake, appetite, and energy expenditure after 10 wk of supplementation in overweight subjects. American Journal of Clinical Nutrition 2014;100(1):36-45. - PubMed
Taljaard 2013 {published data only}
-
- Taljaard C, Covic NM, Graan AE, Kruger HS, Smuts CM, Baumgartner J, et al. Effects of a multi-micronutrient-fortified beverage, with and without sugar, on growth and cognition in South African schoolchildren: a randomised, double-blind, controlled intervention. British Journal of Nutrition 2013;110(12):2271-84. - PubMed
Tsapok 2012 {published data only}
-
- Tsapok PI, Imbriakov KV, Chuchkova MR. Sugar substitute products impact on oral fluid biochemical properties. Stomatologiia 2012;91(2):23-5. - PubMed
Tuttas 2012 {published data only}
-
- Tuttas K, Kirch W. Steviol glycosides as sweetener in diabetes? Deutsche Medizinische Wochenschrift 2012;137(15):806. - PubMed
Vazquez Duran 2013 {published data only}
-
- Vazquez Duran M, Castillo Martinez L, Orea Tejada A, Tellez Olvera DA, Delgado Perez LG, Marquez Zepeda B, et al. Effect of decreasing the consumption of sweetened caloric and non-caloric beverages on weight, body composition and blood pressure in young adults. European Journal of Preventive Cardiology 2013;20(1 Suppl 1):S120.
Verspohl 2014 {published data only}
-
- Verspohl EJ. Type 2 diabetes mellitus: importance of sugar and sugar substitutes. Medizinische Monatsschrift fur Pharmazeuten 2014;37(5):191-2. - PubMed
Vorster 1987 {published data only}
-
- Vorster HH, Tonder E, Kotze JP, Walker AR. Effects of graded sucrose additions on taste preference, acceptability, glycemic index, and insulin response to butter beans. American Journal of Clinical Nutrition 1987;45(3):575-9. - PubMed
Watal 2014 {published data only}
Williams 1857 {published data only}
-
- Williams T, Lond MD. On the effects of saccharine diet in diabetes mellitus. British Medical Journal 1857;s4-1(51):1041-2.
Williams 1858 {published data only}
-
- Williams T. Saccharine diet in diabetes. British Medical Journal 1858;s4-1(53):18.
Williams 2014 {published data only}
-
- Williams O. Botanicals in diabetes treatment: a look at stevia. drugtopics.modernmedicine.com/drug-topics/content/tags/diabetes/botanica... (accessed 27 January 2018).
Wills 1981 {published data only}
-
- Wills JH, Serrone DM, Coulston F. A 7-month study of ingestion of sodium cyclamate by human volunteers. Regulatory Toxicology and Pharmacology : RTP 1981;1(2):163-76.
Ylikahri 1980 {published data only}
-
- Ylikahri R, Pelkonen R. Artificial sweeteners in the diabetic diet. Duodecim 1980;96(9):659-62. - PubMed
Zöllner 1971 {published data only}
-
- Zöllner N, Pieper M. Concluding report of a 3-year clinical study on cyclamate. Arzneimittel-Forschung 1971;21(3):431-2. - PubMed
Additional references
Abrams 2005
-
- Abrams KR, Gillies CL, Lambert PC. Meta-analysis of heterogeneously reported trials assessing change from baseline. Statistics in Medicine 2005;24:3823-44. - PubMed
ADA 2003
-
- Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 2003;26(Suppl 1):S5-20. - PubMed
ADA 2008
-
- American Diabetes Association. Standards of medical care in diabetes - 2008. Diabetes Care 2008;31(Suppl 1):S12-54. [PMID: ] - PubMed
ADA 2016
-
- American Diabetes Association. Obesity management for the treatment of type 2 diabetes. Diabetes Care 2016;39(Suppl 1):S47–51. - PubMed
Aguilar 2007
-
- Aguilar F, Autrup H, Barlow S, Castle L, Crebelli R, Dekant W, et al. Neotame as a sweetener and flavour enhancer. Scientific opinion of the panel on food additives, flavourings, processing aids and materials in contact with food. EFSA Journal 2007;581:1-43.
Altman 2003
Azad 2017
-
- Azad MB, Abou-Setta AM, Chauhan BF, Rabbani R, Lys J, Copstein L, et al. Nonnutritive sweeteners and cardiometabolic health: a systematic review and meta-analysis of randomized controlled trials and prospective cohort studies. CMAJ : Canadian Medical Association Journal 2017;189(28):E929-39. - PMC - PubMed
Bantle 1986
-
- Bantle JP, Laine DC, Thomas JW. Metabolic effects of dietary fructose and sucrose in types I and II diabetic subjects. JAMA 1986;256:3241-6. - PubMed
Bell 2013
-
- Bell ML, McKenzie JE. Designing psycho-oncology randomised trials and cluster randomised trials: variance components and intra-cluster correlation of commonly used psychosocial measures. Psycho-Oncology 2013;22:1738-47. - PubMed
Borenstein 2017a
-
- Borenstein M, Higgins JP, Hedges LV, Rothstein HR. Basics of meta-analysis: I² is not an absolute measure of heterogeneity. Research Synthesis Methods 2017;8(1):5-18. - PubMed
Borenstein 2017b
-
- Borenstein M. Prediction intervals. www.meta-analysis.com/prediction (accessed 3 July 2017).
Boutron 2014
-
- Boutron I, Altman DG, Hopewell S, Vera-Badillo F, Tannock I, Ravaud P. Impact of spin in the abstracts of articles reporting results of randomized controlled trials in the field of cancer: the SPIIN randomized controlled trial. Journal of Clinical Oncology 2014;32:4120-6. - PubMed
Ceunen 2013
-
- Ceunen S, Geuns JM. Steviol glycosides: chemical diversity, metabolism, and function. Journal of Natural Products 2013;76(6):1201-28. - PubMed
Chattopadhyay 2014
Cochrane 2018
-
- Cochrane. CENTRAL creation details. www.cochranelibrary.com/central/central-creation (accessed 21 August 2018).
CONSORT 2010
-
- Schulz KF, Altman DG, Moher D, for the CONSORT Group. CONSORT 2010 Statement: Updated Guidelines for Reporting Parallel Group Randomized Trials. Annals of Internal Medicine 2010;152(11):726-32. - PubMed
Corbett 2014
-
- Corbett MS, Higgins JP, Woolacott NF. Assessing baseline imbalance in randomised trials: implications for the Cochrane risk of bias tool. Research Synthesis Methods 2014;5:79-85. - PubMed
Coulston 1985
-
- Coulston AM, Hollenbeck CB, Donner CC, Williams R, Chiou YA, Reaven GM. Metabolic effects of added dietary sucrose in individuals with noninsulin-dependent diabetes mellitus (NIDDM). Metabolism 1985;34:962-6. - PubMed
Coulston 1987
-
- Coulston AM, Hollenbeck CB, Swislocki AL, Chen YD, Reaven GM. Deleterious metabolic effects of high-carbohydrate, sucrose-containing diets in patients with non-insulin-dependent diabetes mellitus. American Journal of Medicine 1987;82:213-20. - PubMed
Covidence [Computer program]
-
- Veritas Health Innovation Covidence. Version accessed 19 October 2017. Melbourne, Australia: Veritas Health Innovation.Available at covidence.org.
Deeks 2019
-
- Deeks JJ, Higgins JP, Altman DG (editors). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
EC 2011
-
- European Commission. Commission regulation (EU) No 1131/2011 of 11 November 2011 amending annex II to regulation (EC) No 1333/2008 of the European Parliament and of the council with regard to steviol glycosides. eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2011:295:0205:0211:E... (accessed 19 October 2017).
Evert 2013
FDA 2008
-
- US Food and Drug Administration. Agency response letter GRAS notice no. GRN 000252. www.fda.gov/Food/IngredientsPackagingLabeling/GRAS/NoticeInventory/ucm15... (accessed 19 October 2017).
FDA 2015a
-
- US Food and Drug Administration. High-intensity sweeteners. www.fda.gov/food/ingredientspackaginglabeling/foodadditivesingredients/u... (accessed 26 January 2017).
FDA 2015b
-
- US Food and Drug Administration. Additional information about high-intensity sweeteners permitted for use in food in the United States. www.fda.gov/food/ingredientspackaginglabeling/foodadditivesingredients/u... (accessed 19 October 2017).
Fitch 2012
-
- Fitch C, Keim KS, Academy of Nutrition and Dietetics. Position of the Academy of Nutrition and Dietetics: use of nutritive and nonnutritive sweeteners. Journal of the Academy of Nutrition and Dietetics 2012;112(5):739-58. - PubMed
Follmann 1992
-
- Follmann D, Elliott P, Suh I, Cutler J. Variance imputation for overviews of clinical trials with continuous response. Journal of Clinical Epidemiology 1992;45:769-73. - PubMed
FSA 2016
-
- Food Standards Agency. Current EU approved additives and their E numbers. www.food.gov.uk/science/additives/enumberlist#toc-4 (accessed 26 January 2017).
Gallus 2007
-
- Gallus S, Scotti L, Negri E, Talamini R, Franceschi S, Montella M, et al. Artificial sweeteners and cancer risk in a network of case–control studies. Annals of Oncology 2007;18:40-4. - PubMed
Gardner 2012
-
- Gardner C, Wylie-Rosett J, Gidding SS, Steffen LM, Johnson RK, Reader D, et al, American Heart Association Nutrition Committee of the Council on Nutrition, Physical Activity and Metabolism, Council on Arteriosclerosis, Thrombosis and Vascular Biology, Council on Cardiovascular Disease in the Young, American Diabetes Association. Nonnutritive sweeteners: current use and health perspectives: a scientific statement from the American Heart Association and the American Diabetes Association. Diabetes Care 2012;35(8):1798-808. - PMC - PubMed
Greenwood 2014
-
- Greenwood DC, Threapleton DE, Evans CEL, Cleghorn CL, Nykjaer C, Woodhead C, et al. Association between sugar-sweetened and artificially sweetened soft drinks and type 2 diabetes: systematic review and dose–response meta-analysis of prospective studies. British Journal of Nutrition 2014;112(5):725-34. - PubMed
Higgins 2002
-
- Higgins JT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine 2002;21:1539-58. - PubMed
Higgins 2003
Higgins 2009
Higgins 2019a
-
- Higgins JP, Li T, Deeks JJ (editors). Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Higgins 2019b
-
- Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Hoffmann 2014
-
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348:g1687. - PubMed
Hoffmann 2017
-
- Hoffmann TC, Oxman AD, Ioannidis JP, Moher D, Lasserson TJ, Tovey DI, et al. Enhancing the usability of systematic reviews by improving the consideration and description of interventions. BMJ 2017;358:j2998. - PubMed
Hróbjartsson 2013
-
- Hróbjartsson A, Thomsen AS, Emanuelsson F, Tendal B, Hilden J, Boutron I, et al. Observer bias in randomized clinical trials with measurement scale outcomes: a systematic review of trials with both blinded and nonblinded assessors. Canadian Medical Association Journal 2013;185(4):E201-11. - PMC - PubMed
Ilbäck 2003
-
- Ilbäck NG, Alzin M, Jahrl S, Enghardt-Barbieri H, Busk L. Estimated intake of the artificial sweeteners acesulfame-K, aspartame, cyclamate and saccharin in a group of Swedish diabetics. Food Additives and Contaminants 2003;20(2):99-114. - PubMed
JECFA 1982
-
- Joint FAO/WHO Expert Committee on Food Additives. Evaluation of certain food additives and contaminants. World Health Organization; 1982 April, WHO Technical Report Series No.: 683.
JECFA 2004
-
- Joint FAO/WHO Expert Committee on Food Additives (JECFA). Compendium of food additive specifications: Addendum 12. In: WHO Technical Report Series. 63rd Meeting 2004 June 8–17; Geneva. Geneva, Switzerland: World Health Organization, 2004.
JECFA 2010
-
- World Health Organization. Evaluations of the Joint FAO/WHO expert committee on food additives. apps.who.int/food-additives-contaminants-jecfa-database/search.aspx?fcc=1 (accessed 26 January 2017).
Jones 2015
Just 2008
-
- Just T, Pau HW, Engel U, Hummel T. Cephalic phase insulin release in healthy humans after taste stimulation? Appetite 2008;51(3):622-7. - PubMed
Kirkham 2010
-
- Kirkham JJ, Dwan KM, Altman DG, Gamble C, Dodd S, Smyth R, et al. The impact of outcome reporting bias in randomised controlled trials on a cohort of systematic reviews. BMJ 2010;340:c365. - PubMed
Liberati 2009
Lundh 2017
Mathieu 2009
-
- Mathieu S, Boutron I, Moher D, Altman DG, Ravaud P. Comparison of registered and published primary outcomes in randomized controlled trials. JAMA 2009;302:977-84. - PubMed
Mattes 2009
Meader 2014
Mortensen 2006
-
- Mortensen A. Sweeteners permitted in the European Union: safety aspects. Scandinavian Journal of Food and Nutrition 2006;50(3):104-16.
National Diabetes Data Group 1979
-
- National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes 1979;28(12):1039-57. - PubMed
NCD‐RisC 2016
Otabe 2011
-
- Otabe A, Fujieda T, Masuyama T, Ubukata K, Lee C. Advantame - an overview of the toxicity data. Food and Chemical Toxicology 2011;49(Suppl 1):S2-7. - PubMed
Pastors 2002
-
- Pastors JG, Warshaw H, Daly A, Franz M, Kulkarni K. The evidence for the effectiveness of medical nutrition therapy in diabetes management. Diabetes Care 2002;25(3):608-13. - PubMed
Peterson 1986
-
- Peterson DB, Lambert J, Gerring S, Darling P, Carter RD, Jelfs R, et al. Sucrose in the diet of diabetic patients - just another carbohydrate? Diabetologia 1986;29:216-20. - PubMed
Review Manager 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Riley 2011
-
- Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta-analyses. BMJ 2011;342:d549. - PubMed
Romo‐Romo 2016
-
- Romo-Romo A, Aguilar-Salinas CA, Brito-Córdova GX, Gómez Díaz RA, Vilchis Valentín D, Almeda-Valdes P. Effects of the non-nutritive sweeteners on glucose metabolism and appetite regulating hormones: systematic review of observational prospective studies and clinical trials. PLOS ONE 2016;18(11):e0161264. - PMC - PubMed
Scherer 2018
Schünemann 2019
-
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the confidence in or quality of the evidence. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors), Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Sterne 2011
-
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011;343:d4002. - PubMed
Sterne 2017
-
- Sterne JA, Egger M, Moher D, Boutron I, editor(s). Chapter 10: Addressing reporting biases. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Timpe Behnen 2013
-
- Timpe Behnen EM, Ferguson MC, Carlson A. Do sugar substitutes have any impact on glycemic control in patients with diabetes? Journal of Pharmacy Technology 2013;29:61-5.
Toeller 1993
-
- Toeller M. Diet and diabetes. Diabetes/Metabolism Reviews 1993;9:93-108. - PubMed
Toews 2019
WHO 1998
-
- Alberti KM, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part I: diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabetic Medicine 1998;15(7):539-53. - PubMed
WHO 2016
-
- World Health Organization. Diabetes. Fact Sheet (Reviewed November 2016). www.who.int/mediacentre/factsheets/fs312/en (accessed 5 January 2017).
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

