Peptide vaccine as an adjuvant therapy for glypican-3-positive hepatocellular carcinoma induces peptide-specific CTLs and improves long prognosis
- PMID: 32449239
- PMCID: PMC7419030
- DOI: 10.1111/cas.14497
Peptide vaccine as an adjuvant therapy for glypican-3-positive hepatocellular carcinoma induces peptide-specific CTLs and improves long prognosis
Abstract
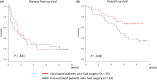
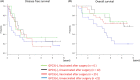
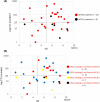
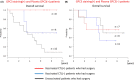
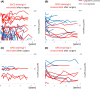
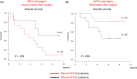
There is no established postoperative adjuvant therapy for hepatocellular carcinoma (HCC), and improvement of patient prognosis has been limited. We conducted long-term monitoring of patients within a phase II trial that targeted a cancer antigen, glypican-3 (GPC3), specifically expressed in HCC. We sought to determine if the GPC3 peptide vaccine was an effective adjuvant therapy by monitoring disease-free survival and overall survival. We also tracked GPC3 immunohistochemical (IHC) staining, CTL induction, and postoperative plasma GPC3 for a patient group that was administered the vaccine (n = 35) and an unvaccinated patient group that underwent surgery only (n = 33). The 1-y recurrence rate after surgery was reduced by approximately 15%, and the 5-y and 8-y survival rates were improved by approximately 10% and 30%, respectively, in the vaccinated group compared with the unvaccinated group. Patients who were positive for GPC3 IHC staining were more likely to have induced CTLs, and 60% survived beyond 5 y. Vaccine efficacy had a positive relationship with plasma concentration of GPC3; high concentrations increased the 5-y survival rate to 75%. We thus expect GPC3 vaccination in patients with HCC, who are positive for GPC3 IHC staining and/or plasma GPC3 to induce CTL and have significantly improved long-term prognosis.
Keywords: cytotoxic T lymphocyte; glypican-3; hepatocellular carcinoma; immunohistochemical staining; peptide vaccine.
© 2020 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
Tetsuya Nakatsura is a founder of and current shareholder at Killer T Save You Co., Ltd. TN is currently receiving royalties from Onco Therapy Science, Inc and fundamental research funding support from Thyas Co., Ltd. and Sysmex Co., Ltd. The remaining authors declare that they have no commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Bosetti C, Turati F, La Vecchia C. Hepatocellular carcinoma epidemiology. Best Pract Res Clin Gastroenterol. 2014;28:753‐770. - PubMed
-
- Carrat F, Fontaine H, Dorival C, et al. Clinical outcomes in patients with chronic hepatitis C after direct‐acting antiviral treatment: a prospective cohort study. Lancet. 2019;393:1453‐1464. - PubMed
-
- Tokushige K, Hyogo H, Nakajima T, et al. Hepatocellular carcinoma in Japanese patients with nonalcoholic fatty liver disease and alcoholic liver disease: multicenter survey. J Gastroenterol. 2016;51:586‐596. - PubMed
-
- Schutte K, Bornschein J, Malfertheiner P. Hepatocellular carcinoma‐epidemiological trends and risk factors. Dig Dis. 2009;27:80‐92. - PubMed
-
- Hanazaki K, Kajikawa S, Shimozawa N. Survival and recurrence after hepatic resection of 386 consecutive patients with hepatocellular carcinoma. J Am Coll Surg. 2000;191:381‐388. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

