Global real-world evidence of sofosbuvir/velpatasvir as simple, effective HCV treatment: Analysis of 5552 patients from 12 cohorts
- PMID: 32449966
- PMCID: PMC7496473
- DOI: 10.1111/liv.14537
Global real-world evidence of sofosbuvir/velpatasvir as simple, effective HCV treatment: Analysis of 5552 patients from 12 cohorts
Abstract
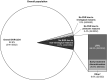
Background and aims: Achieving sustained virological response (SVR; cure) in hepatitis C patients using a simple regimen is key to making elimination by 2030 possible. In the largest real-world analysis to date, the effectiveness of pangenotypic, panfibrotic, single-tablet, sofosbuvir/velpatasvir (SOF/VEL) once-daily for 12 weeks was assessed in 12 clinical real-world cohorts from various geographical areas, settings and treatment practices. Factors affecting risk of not achieving SVR were assessed.
Methods: Adults treated with SOF/VEL 400/100 mg, without ribavirin, were included. All HCV patients reaching Week 12 or 24 post-treatment were assessed for SVR12/24. Factors associated with not achieving SVR12/24 for virological reasons were evaluated using logistic regression analysis.
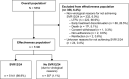
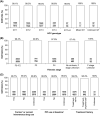
Results: Overall, 5552 patients were included: 13.3% treatment-experienced; 20.7% compensated cirrhotic; 30.2% genotype 1; 29.5% genotype 2; 32.9% genotype 3; 4.7% genotype 4; 3.7% HIV coinfection; 13.4% current/former intravenous drug use. Of the 5196 patients evaluated for effectiveness, 98.9% achieved SVR12/24. High SVR12/24 rates occurred in all genotypes including genotype 3 (98.3%; 1649/1677) and in those with compensated cirrhosis (97.9; 1055/1078). Only 55 patients did not achieve SVR12/24 due to a virological reason; the only factor statistically significantly associated with an increased risk of not achieving SVR12/24 was compensated cirrhosis (P = .002). Overall, 6% (332/5552) of patients did not achieve SVR12/24 for non-virological reasons (67% lost to follow-up; 26.5% early treatment discontinuation).
Conclusions: In this large cohort, representative of clinical practice, a simple 12-week regimen of SOF/VEL without ribavirin resulted in high SVR12/24 rates in diverse patient populations, even among those with compensated cirrhosis.
Keywords: HCV elimination; Hepatitis C; heterogenous; real-world; sofosbuvir/velpatasvir; sustained virological response.
© 2020 The Authors. Liver International published by John Wiley & Sons Ltd.
Conflict of interest statement
Alessandra Mangia: Research grants from Gilead Sciences, Merck Sharp and Dohme, Janssen and Bristol‐Myers Squibb. Part of the speaker bureau for Gilead Sciences, Intercept and Merck Sharp and Dohme. Scott Milligan: Research support from Gilead Sciences, during the conduct of the study. Research support from Gilead Sciences, AbbVie and Merck Sharp and Dohme, outside the submitted work. Mandana Khalili: Grants from HCV‐TARGET study sponsors, during the conduct of the study. HCV‐TARGET is an investigator‐initiated study jointly sponsored by The University of Florida, Gainesville, FL (PI: Nelson), and The University of North Carolina at Chapel Hill, Chapel Hill, NC (PI: Fried) and is funded in part by AbbVie, Bristol Myers Squibb, Gilead, GlaxoSmithKline, Janssen, Kadmon, and Merck. Grants from Gilead Sciences, Intercept Pharmaceuticals and AbbVie, outside of submitted work. Personal fees from Gilead Sciences, outside the submitted work. Stefano Fagiuoli: Grants and personal fees from Gilead Sciences, AbbVie and Novartis, outside the submitted work. Personal fees from Merck Sharp and Dohme, Astellas and Bayer, outside the submitted work. Stephen Shafran: Grants and personal fees from Gilead Sciences, during the conduct of the study. Grants from AbbVie, Merck Sharp and Dohme, and Janssen, outside the submitted work. Fabrice Carrat; Grants from Inserm‐ANRS, during the conduct of the study. Denis Ouzan: Grants from Gilead Sciences, during the conduct of the study. Grants, personal fees and non‐financial support from Gilead Sciences and AbbVie, outside the submitted work. George Papatheodoridis: Grants from Gilead Sciences, during the conduct of the study. Grants from Gilead Sciences and AbbVie, outside of submitted work. Personal fees and non‐financial support from Gilead Sciences, AbbVie and Merck Sharp and Dohme, outside of submitted work. Alnoor Ramji: Grants from AbbVie, Gilead Sciences, Janssen, Novartis, Merck and Springbanks, outside the submitted work. Other from AbbVie, Allergen, Assembly, Celgene, Gilead Sciences and Janssen. Sergio Borgia: Nothing to disclose. Heiner Wedemeyer: Grants and personal fees from Gilead Sciences, during the conduct of the study. Grants, personal fees and non‐financial support from Abbott and Roche Diagnostics, outside the submitted work. Grants and personal fees from Bristol Myers Squibb, Novartis, Roche Diagnostics, Merck Sharp and Dohme, Eiger and Falk and Falk Foundation, outside the submitted work. Personal fees from Siemens and Janssen, outside the submitted work. Investigator for Transgene, outside the submitted work. Non‐financial support from MYR‐GmbH, outside the submitted work. Ruggero Losappio: Nothing to disclose. Francisco Pérez‐Hernandez: Personal fees from Gilead Sciences and AbbVie, outside the submitted work. Nicole Wick: Research support from Gilead Sciences, during the conduct of the study. Research support from AbbVie and Merck, outside the submitted work. Robert S Brown Jr: Grants and personal fees from Gilead Sciences, during the conduct of the study. Grants and personal fees from AbbVie, outside of submitted work. Pietro Lampertico: Personal fees from Gilead Sciences, AbbVie and Merck Sharp and Dohme, outside the submitted work. Karen Doucette: Clinical trials and honoraria from Gilead Sciences and unrestricted educational grant from Merck, outside the submitted work. Ioanna Ntalla, Heribert Ramroth, Michael Mertens and Kim Vanstraelen: Employees and stock owners of Gilead Sciences, during the conduct of the study and outside of submitted work. Juan Turnes: Personal fees from AbbVie, outside the submitted work. Personal fees and grant from Gilead Sciences, outside the submitted work.
Figures
References
-
- World Health Organization . Global health sector strategy on viral hepatitis, 2016–2021: towards ending viral hepatitis. 2016. https://apps.who.int/iris/bitstream/handle/10665/246177/WHO‐HIV‐2016.06‐.... Accessed April 2020
-
- European Association for the Study of the Liver . EASL recommendations on treatment of hepatitis C 2018. J Hepatol. 2018;69:461‐511. - PubMed
-
- Belli LS, Perricone G, Adam R, et al. Impact of DAAs on liver transplantation: major effects on the evolution of indications and results. An ELITA study based on the ELTR registry. J Hepatol. 2018;69:810‐817. - PubMed
-
- Carrat F, Fontaine H, Dorival C, et al. Clinical outcomes in patients with chronic hepatitis C after direct‐acting antiviral treatment: a prospective cohort study. Lancet. 2019;393:1453‐1464. - PubMed
-
- World Health Organization . Guidelines for the care and treatment of persons diagnosed with chronic hepatitis C virus infection. 2018. https://apps.who.int/iris/bitstream/handle/10665/273174/9789241550345‐en.... Accessed April 2020 - PubMed

