RETRACTED: Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis
- PMID: 32450107
- PMCID: PMC7255293
- DOI: 10.1016/S0140-6736(20)31180-6
RETRACTED: Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis
Erratum in
-
Department of Error.Lancet. 2020 May 30:S0140-6736(20)31249-6. doi: 10.1016/S0140-6736(20)31249-6. Online ahead of print. Lancet. 2020. PMID: 32485145 Free PMC article. No abstract available.
Retraction in
-
Retraction-Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis.Lancet. 2020 Jun 13;395(10240):1820. doi: 10.1016/S0140-6736(20)31324-6. Epub 2020 Jun 5. Lancet. 2020. PMID: 32511943 Free PMC article. No abstract available.
-
Retraction and republication: cardiac toxicity of hydroxychloroquine in COVID-19.Lancet. 2020 Jul 18;396(10245):e2-e3. doi: 10.1016/S0140-6736(20)31528-2. Epub 2020 Jul 9. Lancet. 2020. PMID: 32653079 Free PMC article. No abstract available.
Expression of concern in
-
Expression of concern: Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis.Lancet. 2020 Jun 13;395(10240):e102. doi: 10.1016/S0140-6736(20)31290-3. Epub 2020 Jun 3. Lancet. 2020. PMID: 32504543 Free PMC article. No abstract available.
Abstract
Background: Hydroxychloroquine or chloroquine, often in combination with a second-generation macrolide, are being widely used for treatment of COVID-19, despite no conclusive evidence of their benefit. Although generally safe when used for approved indications such as autoimmune disease or malaria, the safety and benefit of these treatment regimens are poorly evaluated in COVID-19.
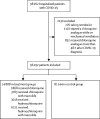
Methods: We did a multinational registry analysis of the use of hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19. The registry comprised data from 671 hospitals in six continents. We included patients hospitalised between Dec 20, 2019, and April 14, 2020, with a positive laboratory finding for SARS-CoV-2. Patients who received one of the treatments of interest within 48 h of diagnosis were included in one of four treatment groups (chloroquine alone, chloroquine with a macrolide, hydroxychloroquine alone, or hydroxychloroquine with a macrolide), and patients who received none of these treatments formed the control group. Patients for whom one of the treatments of interest was initiated more than 48 h after diagnosis or while they were on mechanical ventilation, as well as patients who received remdesivir, were excluded. The main outcomes of interest were in-hospital mortality and the occurrence of de-novo ventricular arrhythmias (non-sustained or sustained ventricular tachycardia or ventricular fibrillation).
Findings: 96 032 patients (mean age 53·8 years, 46·3% women) with COVID-19 were hospitalised during the study period and met the inclusion criteria. Of these, 14 888 patients were in the treatment groups (1868 received chloroquine, 3783 received chloroquine with a macrolide, 3016 received hydroxychloroquine, and 6221 received hydroxychloroquine with a macrolide) and 81 144 patients were in the control group. 10 698 (11·1%) patients died in hospital. After controlling for multiple confounding factors (age, sex, race or ethnicity, body-mass index, underlying cardiovascular disease and its risk factors, diabetes, underlying lung disease, smoking, immunosuppressed condition, and baseline disease severity), when compared with mortality in the control group (9·3%), hydroxychloroquine (18·0%; hazard ratio 1·335, 95% CI 1·223-1·457), hydroxychloroquine with a macrolide (23·8%; 1·447, 1·368-1·531), chloroquine (16·4%; 1·365, 1·218-1·531), and chloroquine with a macrolide (22·2%; 1·368, 1·273-1·469) were each independently associated with an increased risk of in-hospital mortality. Compared with the control group (0·3%), hydroxychloroquine (6·1%; 2·369, 1·935-2·900), hydroxychloroquine with a macrolide (8·1%; 5·106, 4·106-5·983), chloroquine (4·3%; 3·561, 2·760-4·596), and chloroquine with a macrolide (6·5%; 4·011, 3·344-4·812) were independently associated with an increased risk of de-novo ventricular arrhythmia during hospitalisation.
Interpretation: We were unable to confirm a benefit of hydroxychloroquine or chloroquine, when used alone or with a macrolide, on in-hospital outcomes for COVID-19. Each of these drug regimens was associated with decreased in-hospital survival and an increased frequency of ventricular arrhythmias when used for treatment of COVID-19.
Funding: William Harvey Distinguished Chair in Advanced Cardiovascular Medicine at Brigham and Women's Hospital.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Figures


Comment in
-
Does Adding of Hydroxychloroquine to the Standard Care Provide any Benefit in Reducing the Mortality among COVID-19 Patients?: a Systematic Review.J Neuroimmune Pharmacol. 2020 Sep;15(3):349. doi: 10.1007/s11481-020-09940-9. Epub 2020 Sep 2. J Neuroimmune Pharmacol. 2020. PMID: 32607690 Free PMC article. No abstract available.
-
[Tribulaciones de la investigación en los tiempos de covid-19].Rev Colomb Obstet Ginecol. 2020 Sep;71(3):231-236. doi: 10.18597/rcog.3621. Rev Colomb Obstet Ginecol. 2020. PMID: 33247886 Spanish. No abstract available.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

