FOXO1 contributes to diabetic cardiomyopathy via inducing imbalanced oxidative metabolism in type 1 diabetes
- PMID: 32450616
- PMCID: PMC7348139
- DOI: 10.1111/jcmm.15418
FOXO1 contributes to diabetic cardiomyopathy via inducing imbalanced oxidative metabolism in type 1 diabetes
Abstract
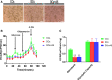
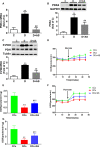
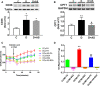
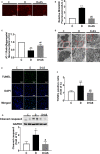
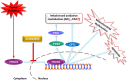
Forkhead box protein O1 (FOXO1), a nuclear transcription factor, is preferably activated in the myocardium of diabetic mice. However, its role and mechanism in the development of diabetic cardiomyopathy in non-obese insulin-deficient diabetes are unclear. We hypothesized that cardiac FOXO1 over-activation was attributable to the imbalanced myocardial oxidative metabolism and mitochondrial and cardiac dysfunction in type 1 diabetes. FOXO1-selective inhibitor AS1842856 was administered to streptozotocin-induced diabetic (D) rats, and cardiac functions, mitochondrial enzymes PDK4 and CPT1 and mitochondrial function were assessed. Primary cardiomyocytes isolated from non-diabetic control (C) and D rats were treated with or without 1 µM AS1842856 and underwent Seahorse experiment to determine the effects of glucose, palmitate and pyruvate on cardiomyocyte bioenergetics. The results showed diabetic hearts displayed elevated FOXO1 nuclear translocation, concomitant with cardiac and mitochondrial dysfunction (manifested as elevated mtROS level and reduced mitochondrial membrane potential) and increased cell apoptosis (all P < .05, D vs C). Diabetic myocardium showed impaired glycolysis, glucose oxidation and elevated fatty acid oxidation and enhanced PDK4 and CPT1 expression. AS1842856 attenuated or prevented all these changes except for glycolysis. We concluded that FOXO1 activation, through stimulating PDK4 and CPT1, shifts substrate selection from glucose to fatty acid and causes mitochondrial and cardiac dysfunction.
Keywords: FOXO1; diabetic cardiomyopathy; oxidative metabolism.
© 2020 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors confirm that there are no conflicts of interest regarding the publication of this article.
Figures

References
-
- Varma U, Koutsifeli P, Benson VL, et al. Molecular mechanisms of cardiac pathology in diabetes ‐ Experimental insights. Biochim Biophys Acta‐Mol Basis Dis. 2018;1864(5):1949‐1959. - PubMed
-
- Herrero P, Peterson LR, McGill JB, et al. Increased myocardial fatty acid metabolism in patients with type 1 diabetes mellitus. J Am Coll Cardiol. 2006;47(3):598‐604. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

