Cross-hemispheric gamma synchrony between prefrontal parvalbumin interneurons supports behavioral adaptation during rule shift learning
- PMID: 32451483
- PMCID: PMC7347248
- DOI: 10.1038/s41593-020-0647-1
Cross-hemispheric gamma synchrony between prefrontal parvalbumin interneurons supports behavioral adaptation during rule shift learning
Abstract
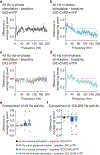
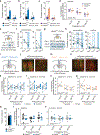
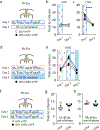
Organisms must learn new strategies to adapt to changing environments. Activity in different neurons often exhibits synchronization that can dynamically enhance their communication and might create flexible brain states that facilitate changes in behavior. We studied the role of gamma-frequency (~40 Hz) synchrony between prefrontal parvalbumin (PV) interneurons in mice learning multiple new cue-reward associations. Voltage indicators revealed cell-type-specific increases of cross-hemispheric gamma synchrony between PV interneurons when mice received feedback that previously learned associations were no longer valid. Disrupting this synchronization by delivering out-of-phase optogenetic stimulation caused mice to perseverate on outdated associations, an effect not reproduced by in-phase stimulation or out-of-phase stimulation at other frequencies. Gamma synchrony was specifically required when new associations used familiar cues that were previously irrelevant to behavioral outcomes, not when associations involved new cues or for reversing previously learned associations. Thus, gamma synchrony is indispensable for reappraising the behavioral salience of external cues.
Figures



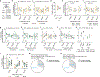


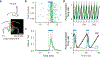
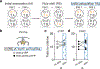
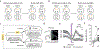

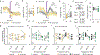


References
-
- Young DA, Davila R, & Scher H. Unawareness of illness and neuropsychological performance in chronic schizophrenia. Schizophr. Res. 10, 117–124 (1993). - PubMed
-
- Shad MU, Tamminga CA, Cullum M, Haas GL, & Keshavan MS Insight and frontal cortical function in schizophrenia: a review. Schizophr. Res. 86, 54–70 (2006). - PubMed
-
- von der Malsburg C. The correlation theory of brain function. Internal Report 81–82, Dept. Neurobiology, Max-Planck-Institute for Biophysical Chemistry (1981).
-
- Abeles M, Prut Y, Bergman H, & Vaadia E. Synchronization in neuronal transmission and its importance for information processing. Prog. Brain Res. 102, 395–404 (1994). - PubMed
-
- Singer W. Neuronal synchrony: a versatile code for the definition of relations? Neuron 49, 111–125 (1999). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

