Hypoxia Pretreatment Promotes Chondrocyte Differentiation of Human Adipose-Derived Stem Cells via Vascular Endothelial Growth Factor
- PMID: 32451775
- PMCID: PMC7260353
- DOI: 10.1007/s13770-020-00265-5
Hypoxia Pretreatment Promotes Chondrocyte Differentiation of Human Adipose-Derived Stem Cells via Vascular Endothelial Growth Factor
Abstract
Background: Human adipose tissue-derived stem cells (ADSCs) are attractive multipotent stem cell sources with therapeutic potential in various fields requiring repair and regeneration, such as acute and chronically damaged tissues. ADSC is suitable for cell-based therapy, but its use has been hampered due to poor survival after administration. Potential therapeutic use of ADSC requires mass production of cells through in vitro expansion. Many studies have consistently observed the tendency of senescence by mesenchymal stem cell (MSC) proliferation upon expansion. Hypoxia has been reported to improve stem cell proliferation and survival.
Methods: We investigated the effects of hypoxia pretreatment on ADCS proliferation, migration capacity, differentiation potential and cytokine production. We also analyzed the effects of vascular endothelial growth factor (VEGF) on osteogenic and chondrogenic differentiation of ADSCs by hypoxia pretreatment.
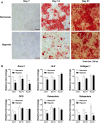
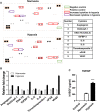
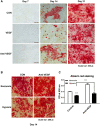
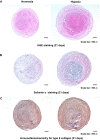
Results: Hypoxia pretreatment increased the proliferation of ADSCs by increasing VEGF levels. Interestingly, hypoxia pretreatment significantly increased chondrogenic differentiation but decreased osteogenic differentiation compared to normoxia. The osteogenic differentiation of ADSC was decreased by the addition of VEGF but increased by the depletion of VEGF. We have shown that hypoxia pretreatment increases the chondrogenic differentiation of ADSCs while reducing osteogenic differentiation in a VEGF-dependent manner.
Conclusion: These results show that hypoxia pretreatment can provide useful information for studies that require selective inhibition of osteogenic differentiation, such as cartilage regeneration.
Keywords: Adipose-derived stem cells (ADSCs); Cartilage regeneration; Differentiation; Hypoxia; VEGF.
Conflict of interest statement
The authors have no conflicts to disclose.
Figures






Similar articles
-
VEGF-mediated proliferation of human adipose tissue-derived stem cells.PLoS One. 2013 Oct 3;8(10):e73673. doi: 10.1371/journal.pone.0073673. eCollection 2013. PLoS One. 2013. PMID: 24098328 Free PMC article.
-
Chemical group-dependent plasma polymerisation preferentially directs adipose stem cell differentiation towards osteogenic or chondrogenic lineages.Acta Biomater. 2017 Mar 1;50:450-461. doi: 10.1016/j.actbio.2016.12.016. Epub 2016 Dec 9. Acta Biomater. 2017. PMID: 27956359 Free PMC article.
-
Substance P enhances proliferation and paracrine potential of adipose-derived stem cells in vitro.Biochem Biophys Res Commun. 2017 Mar 25;485(1):131-137. doi: 10.1016/j.bbrc.2017.02.036. Epub 2017 Feb 10. Biochem Biophys Res Commun. 2017. PMID: 28192115
-
[Multipotential differentiation and potential applications of adipose-derived stem cells].Sheng Wu Gong Cheng Xue Bao. 2007 Mar;23(2):195-200. Sheng Wu Gong Cheng Xue Bao. 2007. PMID: 17460887 Review. Chinese.
-
[Differentiation potential and application of stem cells from adipose tissue].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2012 Aug;26(8):1007-11. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2012. PMID: 23012940 Review. Chinese.
Cited by
-
Hypoxia-inducible factor 2α is a novel inhibitor of chondrocyte maturation.J Cell Physiol. 2021 Oct;236(10):6963-6973. doi: 10.1002/jcp.30356. Epub 2021 Mar 21. J Cell Physiol. 2021. PMID: 33748969 Free PMC article.
-
Extracellular Vesicles Derived From Hypoxia-Conditioned Adipose-Derived Mesenchymal Stem Cells Enhance Lymphangiogenesis.Cell Transplant. 2022 Jan-Dec;31:9636897221107536. doi: 10.1177/09636897221107536. Cell Transplant. 2022. PMID: 35861534 Free PMC article.
-
Adipose Derived Mesenchymal Stem Cells from a Hypoxic Culture Reduce Cartilage Damage.Stem Cell Rev Rep. 2021 Oct;17(5):1796-1809. doi: 10.1007/s12015-021-10169-z. Epub 2021 Apr 24. Stem Cell Rev Rep. 2021. PMID: 33893621
-
Identification of hub genes regulating the cell activity and function of adipose-derived stem cells under oxygen-glucose deprivation.Front Mol Biosci. 2022 Nov 8;9:1025690. doi: 10.3389/fmolb.2022.1025690. eCollection 2022. Front Mol Biosci. 2022. PMID: 36425658 Free PMC article.
-
ADSCs stimulated by VEGF-C alleviate intestinal inflammation via dual mechanisms of enhancing lymphatic drainage by a VEGF-C/VEGFR-3-dependent mechanism and inhibiting the NF-κB pathway by the secretome.Stem Cell Res Ther. 2022 Sep 5;13(1):448. doi: 10.1186/s13287-022-03132-3. Stem Cell Res Ther. 2022. PMID: 36064450 Free PMC article.
References
-
- Dao LT, Park EY, Hwang OK, Cha JY, Jun HS. Differentiation potential and profile of nuclear receptor expression during expanded culture of human adipose tissue-derived stem cells reveals PPARgamma as an important regulator of Oct4 expression. Stem Cells Dev. 2014;23:24–33. - PubMed
-
- Friedenstein AJ, Gorskaja JF, Kulagina NN. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol. 1976;4:267–274. - PubMed
-
- Zhu Y, Liu T, Song K, Fan X, Ma X, Cui Z. Adipose-derived stem cell: a better stem cell than BMSC. Cell Biochem Funct. 2008;26:664–675. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
