Blockade of p38 kinase impedes the mobilization of protumorigenic myeloid populations to impact breast cancer metastasis
- PMID: 32452014
- PMCID: PMC7484223
- DOI: 10.1002/ijc.33050
Blockade of p38 kinase impedes the mobilization of protumorigenic myeloid populations to impact breast cancer metastasis
Abstract
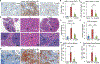
Patients with metastatic breast cancer (MBC) have limited therapeutic options and novel treatments are critically needed. Prior research implicates tumor-induced mobilization of myeloid cell populations in metastatic progression, as well as being an unfavorable outcome in MBC; however, the underlying mechanisms for these relationships remain unknown. Here, we provide evidence for a novel mechanism by which p38 promotes metastasis. Using triple-negative breast cancer models, we showed that a selective inhibitor of p38 (p38i) significantly reduced tumor growth, angiogenesis, and lung metastasis. Importantly, p38i decreased the accumulation of myeloid populations, namely, myeloid-derived suppressor cells (MDSCs) and CD163+ tumor-associated macrophages (TAMs). p38 controlled the expression of tumor-derived chemokines/cytokines that facilitated the recruitment of protumor myeloid populations. Depletion of MDSCs was accompanied by reduced TAM infiltration and phenocopied the antimetastatic effects of p38i. Reciprocally, p38i increased tumor infiltration by cytotoxic CD8+ T cells. Furthermore, the CD163+ /CD8+ expression ratio inversely correlated with metastasis-free survival in breast cancer, suggesting that targeting p38 may improve clinical outcomes. Overall, our study highlights a previously unknown p38-driven pathway as a therapeutic target in MBC.
Keywords: breast cancer; macrophages; metastasis; myeloid-derived suppressor cells; p38 kinase; tumor microenvironment.
© 2020 UICC.
Conflict of interest statement
DISCLOSURE OF INTEREST
The authors report no conflict of interest
Figures
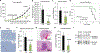




Similar articles
-
Therapeutic targeting with DABIL-4 depletes myeloid suppressor cells in 4T1 triple-negative breast cancer model.Mol Oncol. 2021 May;15(5):1330-1344. doi: 10.1002/1878-0261.12938. Epub 2021 Mar 24. Mol Oncol. 2021. PMID: 33682324 Free PMC article.
-
Folate Receptor Beta Designates Immunosuppressive Tumor-Associated Myeloid Cells That Can Be Reprogrammed with Folate-Targeted Drugs.Cancer Res. 2021 Feb 1;81(3):671-684. doi: 10.1158/0008-5472.CAN-20-1414. Epub 2020 Nov 17. Cancer Res. 2021. PMID: 33203700 Free PMC article.
-
Sphingomyelin synthase 2 facilitates M2-like macrophage polarization and tumor progression in a mouse model of triple-negative breast cancer.Acta Pharmacol Sin. 2021 Jan;42(1):149-159. doi: 10.1038/s41401-020-0419-1. Epub 2020 May 25. Acta Pharmacol Sin. 2021. PMID: 32451413 Free PMC article.
-
Pro-Tumoral Inflammatory Myeloid Cells as Emerging Therapeutic Targets.Int J Mol Sci. 2016 Nov 23;17(11):1958. doi: 10.3390/ijms17111958. Int J Mol Sci. 2016. PMID: 27886105 Free PMC article. Review.
-
TIME Is a Great Healer-Targeting Myeloid Cells in the Tumor Immune Microenvironment to Improve Triple-Negative Breast Cancer Outcomes.Cells. 2020 Dec 23;10(1):11. doi: 10.3390/cells10010011. Cells. 2020. PMID: 33374595 Free PMC article. Review.
Cited by
-
Developmental pathways of myeloid-derived suppressor cells in neoplasia.Cell Immunol. 2021 Feb;360:104261. doi: 10.1016/j.cellimm.2020.104261. Epub 2020 Dec 16. Cell Immunol. 2021. PMID: 33373817 Free PMC article. Review.
-
Thyroid hormones act as modulators of inflammation through their nuclear receptors.Front Endocrinol (Lausanne). 2022 Aug 8;13:937099. doi: 10.3389/fendo.2022.937099. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36004343 Free PMC article. Review.
-
Involvement of p38 MAPK and MAPKAPK2 in promoting cell death and the inflammatory response to ischemic stress associated with necrotic glioblastoma.Cell Death Dis. 2025 Jan 14;16(1):12. doi: 10.1038/s41419-025-07335-3. Cell Death Dis. 2025. PMID: 39805854 Free PMC article.
-
Pharmacological modulation of myeloid-derived suppressor cells to dampen inflammation.Front Immunol. 2022 Aug 30;13:933847. doi: 10.3389/fimmu.2022.933847. eCollection 2022. Front Immunol. 2022. PMID: 36110844 Free PMC article. Review.
-
The Immune Landscape of Breast Cancer: Strategies for Overcoming Immunotherapy Resistance.Cancers (Basel). 2021 Nov 29;13(23):6012. doi: 10.3390/cancers13236012. Cancers (Basel). 2021. PMID: 34885122 Free PMC article. Review.
References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA: A Cancer Journal for Clinicians 2018;68: 7–30. - PubMed
-
- Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, Bonnefoi H, Cameron D, Gianni L, Valagussa P, Swain SM, Prowell T, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. The Lancet 2014;384: 164–72. - PubMed
-
- Saleh SMI, Bertos N, Gruosso T, Gigoux M, Souleimanova M, Zhao H, Omeroglu A, Hallett MT, Park M. Identification of Interacting Stromal Axes in Triple-Negative Breast Cancer. Cancer Research 2017;77: 4673. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

