Temporal association of sNfL and gad-enhancing lesions in multiple sclerosis
- PMID: 32452160
- PMCID: PMC7318095
- DOI: 10.1002/acn3.51060
Temporal association of sNfL and gad-enhancing lesions in multiple sclerosis
Abstract
Objective: Multiple sclerosis (MS) is an autoimmune demyelinating disorder, which is characterized by relapses and remissions. Serum neurofilament light chain (sNfL) is an emerging biomarker of disease activity but its clinical use is still limited. In this study, we aim to characterize the temporal association between sNfL and new clinical relapses and new gadolinium-enhancing (Gd+) lesions.
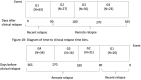
Methods: Annual sNfL levels were measured with a single-molecule array (SIMOA) assay in 94 patients with MS enrolled in the Comprehensive Longitudinal Investigation of Multiple Sclerosis at the Brigham and Women's Hospital (CLIMB) study. We used a multivariable linear mixed-effects model to test the temporal association of sNfL with clinical relapses and/or new Gd+ lesions. We adjusted this model for age, disease duration, sex, and disease-modifying therapies (DMTs) use.
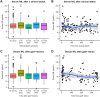
Results: In the 3 months after a Gd+ lesion, we observed an average 35% elevation in sNfL (P < 0.0001) compared to remission samples. We also observed an average 32.3% elevation in sNfL at the time of or prior to a Gd+ lesion (P = 0.002) compared to remission. We observed a significant elevation in sNfL after a clinical relapse only when associated with a Gd+ lesion.
Interpretation: Our findings support sNfL as a marker of clinical relapses and Gd+ lesions. sNfL peaks in a 3-month window around Gd+ lesions. sNfL shows promise as a biomarker of neurological inflammation and possibly of simultaneous Gd+ lesions during a clinical relapse.
© 2020 The Authors. Annals of Clinical and Translational Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
Rosso received support from Verily Life Sciences and Biogen. Gonzalez received research support from Verily Life Sciences. Healy was on the Biogen Worldwide Medical Biostatistics Multiple Sclerosis Advisory Board and received grant support from Genzyme, Merck Serono, and Novartis. Paul: nothing to disclose. Saxena received support from Verily Life Sciences and Biogen. Bjornevik: nothing to disclose. Kuhle received and exclusively used for research support: consulting fees from Biogen, Novartis, Protagen AG, Roche, and Teva; speaker fees from the Swiss MS Society, Biogen, Genzyme, Merck, Novartis, Roche; travel expenses from Merck Serono, Novartis, and Roche; and grants from the ECTRIMS Research Fellowship Programme, University of Basel, Swiss MS Society, Swiss National Research Foundation (320030_160221), Bayer, Biogen, Genzyme, Merck, Novartis, and Roche. Benkert. reports no conflicts of interest. Leppert is an employee of Novartis Pharma AG. Guttmann has received research funding from Sanofi, the National Multiple Sclerosis Society, and the International Progressive Multiple Sclerosis Alliance, the U.S. Office for Naval Research, as well as travel support from Roche Pharmaceuticals; Guttmann owns stock in Roche, Novartis, GSK, Alnylam, Protalix Biotherapeutics, Arrowhead Pharmaceuticals, Cocrystal Pharma, Sangamo Therapeutics. Bakshi has received consulting fees from Bayer, Biogen, Celgene, EMD Serono, Genentech, Guerbet, Sanofi‐Genzyme, and Shire and research support from EMDSerono and Sanofi‐Genzyme. Weiner reports grants from National Institutes of Health, grants from National Multiple Sclerosis Society, Verily Life Sciences, EMD Serono, Biogen, Teva Pharmaceuticals, Sanofi, grants from Novartis, and grants and personal fees from Genentech, Inc, and Tilos Therapeutics, personal fees from Tiziana Life Sciences, IM Therapeutics, MedDay Pharmaceuticals, and vTv Therapeutics, outside the submitted work. Chitnis received personal compensation for advisory board/consulting for Biogen‐Idec, Merck Serono, Novartis, Sanofi, Bayer, Celgene, Alexion and received financial support for research activities from Merck Serono and Novartis Pharmaceuticals. This study was funded in part by EMD Serono.
Figures

References
-
- Confavreux C, Vukusic S. Natural history of multiple sclerosis: a unifying concept. Brain 2006;129:606–616. - PubMed
-
- Schumacher GA, Beebe G, Kibler RF, et al. Problems of experimental trials of therapy in multiple sclerosis: report by the panel on the evaluation of experimental trials of therapy in multiple sclerosis. Ann N Y Acad Sci 1965;122:552–568. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

