Autophagy and post-ischemic conditioning in retinal ischemia
- PMID: 32452260
- PMCID: PMC8205079
- DOI: 10.1080/15548627.2020.1767371
Autophagy and post-ischemic conditioning in retinal ischemia
Abstract
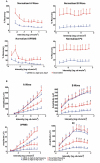
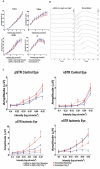
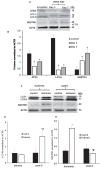
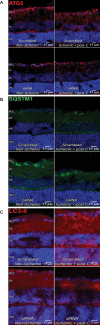
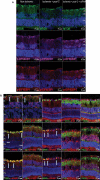
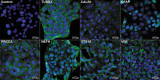
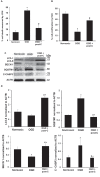
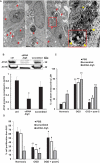
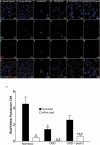
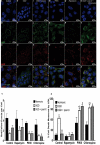
Retinal ischemia is a major cause of vision loss and a common underlying mechanism associated with diseases, such as diabetic retinopathy and central retinal artery occlusion. We have previously demonstrated the robust neuroprotection in retina induced by post-conditioning (post-C), a brief period of ischemia, 24 h, following a prolonged and damaging initial ischemia. The mechanisms underlying post-C-mediated retinal protection are largely uncharacterized. We hypothesized that macroautophagy/autophagy is a mediator of post-C-induced neuroprotection. This study employed an in vitro model of oxygen glucose deprivation (OGD) in the retinal R28 neuronal cell line, and an in vivo rat model of retinal ischemic injury. In vivo, there were significant increases in autophagy proteins, MAP1LC3-II/LC3-II, and decreases in SQSTM1/p62 (sequestosome 1) in ischemia/post-C vs. ischemia/sham post-C. Blockade of Atg5 and Atg7 in vivo decreased LC3-II, increased SQSTM1, attenuated the functional protective effect of post-C, and increased histological damage and TUNEL compared to non-silencing siRNA. TUNEL after ischemia in vivo was found in retinal ganglion, amacrine, and photoreceptor cells. Blockade of Atg5 attenuated the post-C neuroprotection by a brief period of OGD in vitro. Moreover, in vitro, post-C attenuated cell death, loss of cellular proliferation, and defective autophagic flux from prolonged OGD. Stimulating autophagy using Tat-Beclin 1 rescued retinal neurons from cell death after OGD. As a whole, our results suggest that autophagy is required for the neuroprotective effect of retinal ischemic post-conditioning and augmentation of autophagy offers promise in the treatment of retinal ischemic injury.Abbreviations: BECN1: Beclin 1, autophagy related; DAPI: 4',6-diamidino-2-phenylindole; DR: diabetic retinopathy; EdU: 5-ethynyl-2'-deoxyuridine; ERG: Electroretinogram; FITC: Fluorescein isothiocyanate; GCL: Ganglion cell layer; GFAP: Glial fibrillary acidic protein; INL: Inner nuclear layer; IPL: Inner plexiform layer; MAP1LC3/LC3: Microtubule-associated protein 1 light chain 3; OGD: Oxygen-glucose deprivation; ONL: Outer nuclear layer; OP: Oscillatory potential; PFA: Paraformaldehyde; PL: Photoreceptor layer; post-C: post-conditioning; RFP: Red fluorescent protein; RGC: Retinal ganglion cell; RPE: Retinal pigment epithelium; RT-PCR: Real-time polymerase chain reaction; SEM: Standard error of the mean; siRNA: Small interfering RNA; SQSTM1: Sequestosome 1; STR: Scotopic threshold response; Tat: Trans-activator of transcription; TUNEL: Terminal deoxynucleotidyl transferase dUTP nick end labeling.
Keywords: ATG proteins; LC3; MTOR; SQSTM1/p62; TUNEL; autophagic flux; macroautophagy/autophagy; post-ischemic conditioning; retina.
Conflict of interest statement
No potential conflict of interest was reported by the authors.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
