Model of Short- and Long-Term Outcomes of Emicizumab Prophylaxis Treatment for Persons with Hemophilia A
- PMID: 32452276
- PMCID: PMC10391239
- DOI: 10.18553/jmcp.2020.19406
Model of Short- and Long-Term Outcomes of Emicizumab Prophylaxis Treatment for Persons with Hemophilia A
Abstract
Background: Hemophilia A (HA) can result in bleeding events because of low or absent clotting factor VIII (FVIII). Prophylactic treatment for severe HA includes replacement FVIII infusions and emicizumab, a bispecific factor IXa- and factor X-directed antibody.
Objective: To develop an economic model to predict the short- and long-term clinical and economic outcomes of prophylaxis with emicizumab versus short-acting recombinant FVIII among persons with HA in the United States.
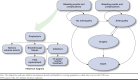
Methods: A Markov model was developed to compare clinical outcomes and costs of emicizumab versus FVIII prophylaxis among persons with severe HA from U.S. payer and societal perspectives. Patients started prophylaxis at age 1 year in the base case. Mutually exclusive health states considered were "no arthropathy," "arthropathy," "surgery," and "death." Serious adverse events, breakthrough bleeds, and inhibitor development were simulated throughout the modeled time horizon. In addition to the prophylaxis drug costs, patients could incur other direct costs related to breakthrough bleeds treatment, serious adverse events, development of inhibitors, arthropathy, and orthopedic surgery. Indirect costs associated with productivity loss (i.e., missed work or disabilities) were applied for adults. Model inputs were obtained from the HAVEN 3 trial, published literature, and expert opinion. The model used a lifetime horizon, and results for 1 year and 5 years were also reported. Deterministic sensitivity analyses and scenario analyses were conducted to assess robustness of the model.
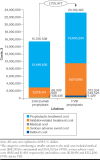
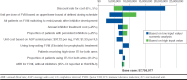
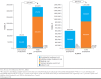
Results: Over a lifetime horizon, the cumulative number of all treated bleeds and joint bleeds avoided on emicizumab versus FVIII prophylaxis were 278.2 and 151.7, respectively. Correspondingly, arthropathy (mean age at onset: 12.9 vs. 5.4 years) and FVIII inhibitor development (mean age at development: 13.9 vs. 1.1 years) were delayed. Total direct and indirect costs were lower for emicizumab versus FVIII prophylaxis for all modeled time horizons ($97,159 vs. $331,610 at 1 year; $603,146 vs. $1,459,496 at 5 years; and $15,238,072 vs. $22,820,281 over a lifetime horizon). The sensitivity analyses indicated that clinical outcomes were sensitive to efficacy inputs, while economic outcomes were driven by the discount rate, dosing schedules, and treatments after inhibitor development. Results for moderate to severe patients were consistent with findings in the severe HA population.
Conclusions: The model suggests that emicizumab prophylaxis confers additional clinical benefits, resulting in a lower number of bleeding events and delayed onset of arthropathy and inhibitor development across all time assessment horizons. Compared with short-acting recombinant FVIII, emicizumab prophylaxis leads to superior patient outcomes and cost savings from U.S. payer and societal perspectives.
Disclosures: Funding for this study was provided by Genentech. Raimundo and Patel are employees of Genentech and own stock or stock options. Zhou, Han, Ji, Fang, Zhong, and Betts are employees of Analysis Group, which received consultancy fees from Genentech for conducting this study. Mahajerin received consultancy fees from Genentech for work on this study. Portions of this research were presented as a poster at the 2018 American Society of Hematology Conference; December 1-4, 2018; San Diego, CA.
Conflict of interest statement
Funding for this study was provided by Genentech. Raimundo and Patel are employees of Genentech and own stock or stock options. Zhou, Han, Ji, Fang, Zhong, and Betts are employees of Analysis Group, which received consultancy fees from Genentech for conducting this study. Mahajerin received consultancy fees from Genentech for work on this study.
Portions of this research were presented as a poster at the 2018 American Society of Hematology Conference; December 1-4, 2018; San Diego, CA.
Figures
References
-
- Centers for Disease Control and Prevention. Data & statistics on hemophilia. Available at: https://www.cdc.gov/ncbddd/hemophilia/data.html. Accessed May 11, 2020.
-
- Riedl J, Ay C, Pabinger I. Platelets and hemophilia: a review of the literature. Thromb Res. 2017;155:131-39. - PubMed
-
- Srivastava A, Brewer AK, Mauser-Bunschoten EP, et al. Guidelines for the management of hemophilia. Haemophilia. 2013;19(1):e1-47. - PubMed
-
- Ljung R, Gretenkort Andersson N. The current status of prophylactic replacement therapy in children and adults with haemophilia. Br J Haematol. 2015;169(6):777-86. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

