Lanadelumab demonstrates rapid and sustained prevention of hereditary angioedema attacks
- PMID: 32452549
- PMCID: PMC7689768
- DOI: 10.1111/all.14416
Lanadelumab demonstrates rapid and sustained prevention of hereditary angioedema attacks
Abstract
Background: Lanadelumab demonstrated efficacy in preventing hereditary angioedema (HAE) attacks in the phase 3 HELP Study.
Objective: To assess time to onset of effect and long-term efficacy of lanadelumab, based on exploratory findings from the HELP Study.
Methods: Eligible patients with HAE type I/II received lanadelumab 150 mg every 4 weeks (q4wks), 300 mg q4wks, 300 mg q2wks, or placebo. Ad hoc analyses evaluated day 0-69 findings using a Poisson regression model accounting for overdispersion. Least-squares mean monthly HAE attack rate for lanadelumab was compared with placebo. Intrapatient comparisons for days 0-69 versus steady state (days 70-182) used a paired t test for continuous endpoints or Kappa statistics for categorical endpoints.
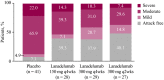
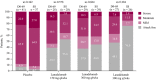
Results: One hundred twenty-five patients were randomized and treated. During days 0-69, mean monthly attack rate was significantly lower with lanadelumab (0.41-0.76) vs placebo (2.04), including attacks requiring acute treatment (0.33-0.61 vs 1.66) and moderate/severe attacks (0.31-0.48 vs 1.33, all P ≤ .001). More patients receiving lanadelumab vs placebo were attack free (37.9%-48.1% vs 7.3%) and responders (85.7%-100% vs 26.8%). During steady state, the efficacy of lanadelumab vs placebo was similar or improved vs days 0-69. Intrapatient differences were significant with lanadelumab 300 mg q4wks for select outcomes. Lanadelumab efficacy was durable-HAE attack rate was consistently lower vs placebo, from the first 2 weeks of treatment through study end. Treatment emergent adverse events were comparable during days 0-69 and 70-182.
Conclusion: Protection with lanadelumab started from the first dose and continued throughout the entire study period.
Keywords: durable efficacy; hereditary angioedema; long-term prophylaxis; onset of action.
© 2020 The Authors. Allergy published by European Academy of Allergy and Clinical Immunology and John Wiley & Sons Ltd.
Conflict of interest statement
MAR has received research grants from BioCryst, CSL Behring, Pharming, and Shire*; consulting fees from Adverum, Attune, BioCryst, CSL Behring, KalVista, Pharming, Pharvaris, and Shire*; and speaker honoraria from CSL Behring, Pharming, and Shire; and is a medical advisory board member of the US Hereditary Angioedema Association. MM has received research grant support and/or speaker/consultancy fees from Adverum, Attune, BioCryst, CSL Behring, KalVista, Pharming, Pharvaris, and Shire.* JAB has been a clinical investigator for BioCryst, CSL Behring, Pharming, and Shire*; a speaker for CSL Behring, Pharming, and Shire*; and a consultant for BioCryst, CSL Behring, Kabi, KalVista, Pharming, and Shire*; and is a medical advisory board member of the US Hereditary Angioedema Association. AB has received institutional research/study support from BioCryst and Shire* and/or honoraria for consulting from BioCryst, CSL Behring, KalVista, Pharming, Pharvaris, and Shire.* HJL has received research grant support and/or speaker/consultancy fees from Adverum, BioCryst, CSL Behring, Octapharma, Pharming, and Shire.* HHL has been a clinical investigator and received grants and/or honoraria from BioCryst, CSL Behring, Pharming, and Shire.* PL was a full‐time employee of Shire* at the time of this analysis and holds stock/stock options in Takeda. Her current affiliation is Pharvaris B.V. JH and SJ are full‐time employees of Shire* and hold stock/stock options in Takeda. WRL has received consultant fees from Adverum, BioCryst, CSL Behring, Pharming, and Shire*; research grants from BioCryst, CSL Behring, Pharming, and Shire*; and payments for lectures from CSL Behring, Pharming, and Shire*; and is a medical advisory board member of the US Hereditary Angioedema Association.
*A Takeda company.
Figures



References
-
- Davis‐Lorton M. An update on the diagnosis and management of hereditary angioedema with abnormal C1 inhibitor. J Drugs Dermatol. 2015;14(2):151‐157. - PubMed
-
- Maurer M, Magerl M, Ansotegui I, et al. The international WAO/EAACI guideline for the management of hereditary angioedema—the 2017 revision and update. Allergy. 2018;73(8):1575‐1596. - PubMed
-
- Johnston DT. Diagnosis and management of hereditary angioedema. J Am Osteopath Assoc. 2011;111(1):28‐36. - PubMed
-
- Banerji A. The burden of illness in patients with hereditary angioedema. Ann Allergy Asthma Immunol. 2013;111(5):329‐336. - PubMed
-
- Bork K, Hardt J, Witzke G. Fatal laryngeal attacks and mortality in hereditary angioedema due to C1‐INH deficiency. J Allergy Clin Immunol. 2012;130(3):692‐697. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

