Strategies for optimising antenatal corticosteroid administration for women with anticipated preterm birth
- PMID: 32452555
- PMCID: PMC7387231
- DOI: 10.1002/14651858.CD013633
Strategies for optimising antenatal corticosteroid administration for women with anticipated preterm birth
Abstract
Background: Preterm birth is a serious and common pregnancy complication. The burden is particularly high in low- and middle-income countries where available care is often inadequate to ensure preterm newborn survival. Administration of antenatal corticosteroids (ACS) is recommended as the standard care for the management of women at risk of imminent preterm birth but its coverage varies globally. Efforts to improve preterm newborn survival have largely been focused on optimising the coverage of ACS use. However, the benefits and harms of such strategies are unclear.
Objectives: To determine the relative benefits and risks of individual patient protocols, health service policies, educational interventions or other strategies which aim to optimise the use of ACS for anticipated preterm birth.
Search methods: We searched Cochrane Pregnancy and Childbirth's Trials Register, ClinicalTrials.gov, the WHO International Clinical Trials Registry Platform (ICTRP) (26 September 2019), and reference lists of retrieved studies.
Selection criteria: We planned to include randomised controlled trials (RCTs), randomised at individual or cluster level, and quasi-randomised trials that assessed strategies to optimise (either by increasing or restricting) the administration of ACS compared with usual care amongst women at risk of preterm birth. Our primary outcomes were perinatal death and a composite outcome of offspring mortality and early or late neurodevelopmental morbidity.
Data collection and analysis: Two review authors independently assessed studies for inclusion. All three review authors independently extracted data and assessed risk of bias. We used narrative synthesis to analyse results, as we were unable to pool data from the included studies. We assessed the certainty of evidence using the GRADE approach.
Main results: We included three cluster-RCTs, all assessing the effects of a multifaceted strategy aiming to promote the use of ACS among women at risk of preterm birth. We did not identify any trials assessing strategies to restrict the use of ACS versus usual care. Two of the included trials assessed use of ACS in high-resource hospital settings. The third trial, the Antenatal Corticosteroid Trial (ACT) was a multi-site trial conducted in rural and semi-urban settings of six low- and middle-income countries in South Asia, sub-Saharan Africa and Central and South America. In two trials, promoting the use of ACS resulted in increased use of ACS, whereas one trial did not find a difference in the rate of ACS administration compared to usual care. Whilst we included three studies, we were unable to pool the data in meta-analysis due to outcomes not being reported across all studies, or outcome results being reported in different ways. The main source of data in this review is from the ACT trial. We assessed the ACT trial as high risk for performance and selective reporting bias. In the protocol for this review, we planned to report all settings and subgroup by low-middle versus high-income countries; these planned analyses were not possible in this version of the review, although adding further studies in future updates may allow us to carry out planned subgroup analyses. The ACT trial was conducted in low-resource settings and reported data on appropriate ACS treatment and inappropriate ACS treatment. Although a strategy of promoting the administration of ACS compared to routine care may increase appropriate ACS treatment (RR 4.34, 95%CI 3.59 to 5.25; 1 study; n = 4389; low-certainty evidence), it may also increase inappropriate ACS treatment (RR 9.11 95%CI 8.04 to 10.33, 1 study, n = 89,237; low-certainty evidence). In low-resource settings, a strategy of promoting the administration of ACS probably increases population level perinatal death by 3 per 1000 infants (risk ratio (RR) 1.11, 95% confidence interval (CI) 1.04 to 1.19; 1 study; n = 100,705; moderate-certainty evidence); stillbirth by 2 per 1000 infants (RR 1.11, 95% CI 1.02 to 1.21; 1 study; n = 100,705; moderate-certainty evidence); and neonatal death before 28 days by 2 per 1000 infants (RR 1.12, 95% CI 1.02 to 1.23; 1 study; n = 100,705; moderate-certainty evidence); may increase the risk for 'suspected' maternal infection or inflammation (RR 1.49, 95% CI 1.32 to 1.68; 1 study; n = 99,742; low-certainty evidence); and make little or no difference to the risk of maternal mortality (RR 1.11, 95% CI 0.64 to 1.92; 1 study; n = 99,742; low-certainty evidence) compared to routine care. Included trials did not report on the composite outcomes offspring mortality, early neurodevelopmental morbidity or late neurodevelopmental morbidity; and offspring mortality or severe neonatal morbidity.
Authors' conclusions: In low-resource settings, a strategy of actively promoting the use of ACS in women at risk of preterm birth may increase ACS use in the target population, but may also carry a substantial risk of unnecessary exposure of ACS to women in whom ACS is not indicated. At the population level, these effects are probably associated with increased risks of stillbirth, perinatal death, neonatal death before 28 days, and maternal infection. The findings of this review support a more conservative approach to clinical protocols and clinical decision-making particularly in low-resource settings, along the lines of the World Health Organization's ACS 2015 recommendations, which take into account both the established clinical efficacy of ACS when used in the correct situation and context, and the possibility of important adverse effects when certain conditions are not met. Given the unanticipated results of the ACT trial, further research on strategies to optimise the use of ACS in low-resource settings is justified.
Trial registration: ClinicalTrials.gov NCT01084096.
Copyright © 2020 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
G Justus Hofmeyr: none known
Anke C Rohwer was partly supported by the Research, Evidence and Development Initiative (READ‐It) project (project number 300342‐104). READ‐It is funded by aid from the UK government; however, the views expressed do not necessarily reflect the UK government’s official policies.
Olufemi T Oladapo: co‐ordinated the development of the WHO recommendations on interventions to improve newborn outcomes published in 2015, and is the Project Co‐ordinator for the WHO ACTION trials.
Figures

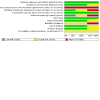
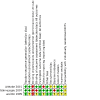








References
References to studies included in this review
Althabe 2015 {published data only}
-
- Althabe F, Belizan JM, McClure EM, Hemingway-Foday J, Berrueta M, Mazzoni A, et al. A population-based, multifaceted strategy to implement antenatal corticosteroid treatment versus standard care for the reduction of neonatal mortality due to preterm birth in low-income and middle-income countries: the ACT cluster-randomised trial. Lancet 2015;385(9968):629-39. - PMC - PubMed
-
- Althabe F, McClure E, Jobe A. Regional use of antenatal corticosteroids and neonatal outcomes in the Global Network Antenatal Corticosteroids Trial (ACT). In: Pediatric Academic Socieities Annual Meeting; 2015 April 25-28; San Diego, California, USA. 2015.
-
- Althabe F. Trial of the use of antenatal corticosteroids in developing countries. clinicaltrials.gov/ct2/show/NCT01084096 (first received: 9 March 2010).
Gülmezoglu 2007 {published data only}ISRCTN14055385
-
- Gülmezoglu AM, Langer A, Piaggoi G, Lumbiganon P, Villar J, Grimshaw J. Cluster randomised trial of an active, multifaceted educational intervention based on the WHO Reproductive Health Library to improve obstetric practices. BJOG: an international journal of obstetrics and gynaecology 2007;114(1):16-23. - PubMed
-
- Gulmezoglu AM, Villar J, Grimshaw J, Piaggio G, Lumbiganon P, Langer A. Cluster randomized trial of an active, multifaceted information dissemination intervention based on the who reproductive health library to change obstetric practices: methods and design issues [isrctn14055385]. BMC Medical Research Methodology 2004;4:2. - PMC - PubMed
-
- ISRCTN14055385. A randomised controlled trial to evaluate a programme promoting evidence-based medicine based on the World Health Organization (WHO) reproductive health library. isrctn.com/ISRCTN14055385 (first received 27 October 2003).
Leviton 1999 {published data only}
-
- Leviton LC, Goldenberg RL, Baker CS, Schwartz RM, Freda MC, Fish LJ, et al. Methods to encourage the use of antenatal corticosteroid therapy for fetal maturation: a randomized controlled trial. JAMA 1999;28(1):46-52. - PubMed
References to studies excluded from this review
McGoldrick 2016 {published data only}
-
- ACTRN12616001210460. A randomised trial comparing semi-structured interviews and online questionnaires administered to health professionals and consumers in identifying the barriers and enablers to implementation of an Antenatal Corticosteroid Clinical Practice Guideline. anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12616001210460 Date first received: 24 August 2016.
-
- Goldrick E, Crawford T, Brown JA, Groom K, Crowther CA. Identifying the barriers and enablers to implementation of the new antenatal corticosteroid clinical practice guidelines among NZ health care professionals using a thematic analysis. Journal of Paediatrics and Child Health 2015;51:A271.
-
- McGoldrick E, Crawford T, Brown JA, Groom KM, Crowther CA. Semi-structured interviews or online questionnaires to identify barriers and enablers to administration of antenatal corticosteroids: a randomised trial. In: Perinatal Society of Australia and New Zealand 20th Annual Conference; 2016 May 22-25; Townsville, Australia. 2016.
Patel 2017 {published data only}
WHO 2019 {published data only}
-
- ACTRN12617000476336. A multi-country, multi-centre, two-arm, parallel, double-blind, placebo-controlled, randomized trial of antenatal corticosteroids for women at risk of imminent birth in the early preterm period in hospitals in low-resource countries to improve newborn outcomes. anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12617000476336 Date first received: 3 February 2017.
-
- The WHO ACTION Trials Collaborators. The World Health Organization ACTION-I (Antenatal Corticosteroids for Improving Outcomes in preterm Newborns) trial—a multi-country, multi-centre, two-arm, parallel, double-blind, placebo-controlled, individually randomized trial of antenatal corticosteroids for women at risk of imminent birth in the early preterm period in hospitals in low-resource countries. Trials 2019;20:507. - PMC - PubMed
Additional references
Bauer 2016
-
- Bauer ST, Price L, MacEachern MP, Housey MT, Langen E, Bauer ME. Maternal leukocytosis after antenatal corticosteroid administration: a systematic review and meta-analysis. Obstetrics and Gynecology 2016;127 Suppl 1:6S. - PubMed
Blencowe 2012
-
- Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, Narwal R, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet 2012;379(9832):2162–72. - PubMed
Boghossain 2016
-
- Boghossian NS, McDonald SA, Bell EF, Carlo WA, Brumbaugh JE, Stoll BJ, et al. Association of antenatal corticosteroids with mortality, morbidity, and neurodevelopmental outcomes in extremely preterm multiple gestation infants. JAMA Pediatrics 2016;170(6):593-601. [DOI: 10.1001/jamapediatrics.2016.0104] - DOI - PMC - PubMed
Braun 2013
-
- Braun T, Husar A, Challis JR, Dudenhausen JW, Henrich W, Plagemann A, et al. Growth restricting effects of a single course of antenatal betamethasone treatment and the role of human placental lactogen. Placenta 2013;34(5):407-15. - PubMed
Chawanpaiboon 2019
Crowther 2015
Crowther 2016
-
- Crowther CA, Harding JE. Antenatal glucocorticoids for late preterm birth? New England Journal of Medicine 2016;374(14):1376-7. - PubMed
Ecker 2016
-
- American College of Obstetricians and Gynecologists and the Society for Maternal–Fetal Medicine, Ecker JL, Kaimal A, Mercer BM, Blackwell SC, deRegnier RA, Farrell RM, et al. Periviable birth: Interim update. American Journal of Obstetrics and Gynecology 2016;215(2):B2-B12.e1. - PubMed
Heneghan 2017
Higgins 2011
-
- Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Institute of Medicine (US) 2007
-
- Institute of Medicine (US) Committee on Understanding Premature Birth and Assuring Healthy Outcomes, Behrman RE, Butler AS, editors. Preterm Birth: Causes, Consequences, and Prevention. Washington (DC): National Academies Press (US), 2007. - PubMed
Jobe 2018
-
- Jobe AH, Goldenberg RL. Antenatal corticosteroids: an assessment of anticipated benefits and potential risks. American Journal of Obstetrics and Gynecology 2018;219(1):62-74. [DOI: ] - PubMed
Liu 2015
-
- Liu L, Oza S, Hogan D, Perin J, Rudan I, Lawn JE, et al. Global, regional, and national causes of child mortality in 2000-13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet 2015;385(9966):430–40. - PubMed
Mandondo 2018
-
- Mandondo SD, Hofmeyr GJ, Mbengo F, Mshweshwe-Paleka NT, Mavundla TR. Outcomes of self-induced late pregnancy termination in women presenting to a tertiary hospital in the Eastern Cape Province, South Africa. South African Medical Journal 2018;108(11):965-71. - PubMed
Massawe 2018
Nada 2016
-
- Nada AM, Shafeek MM, El Maraghy MA, Nageeb AH, Salah El Din AS, Awad MH. Antenatal corticosteroid administration before elective caesarean section at term to prevent neonatal respiratory morbidity: a randomized controlled trial. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2016;199:88-91. - PubMed
Park 2016
-
- Park CK, Isayama T, McDonald SD. Antenatal corticosteroid therapy before 24 weeks of gestation: a systematic review and meta-analysis. Obstetrics and Gynecology 2016;127(4):715-25. - PubMed
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roberts 2017
Saccone 2016
Son 2017
Sotiriadis 2018
van ʼt Hooft 2016
Vogel 2017
-
- Vogel JP, Oladapo OT, Pileggi-Castro C, Adejuyigbe EA, Althabe F, Ariff S, et al. Antenatal corticosteroids for women at risk of imminent preterm birth in low-resource countries: the case for equipoise and the need for efficacy trials. BMJ Global Health 2017;2(3):e000398. doi:10.1136/ bmjgh-2017-000398. - PMC - PubMed
Wapner 2016
WHO 2015
-
- WHO. WHO recommendations on interventions to improve preterm birth outcomes. Switzerland: WHO Press, 2015. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

