An up-to-date overview of computational polypharmacology in modern drug discovery
- PMID: 32452701
- PMCID: PMC7415563
- DOI: 10.1080/17460441.2020.1767063
An up-to-date overview of computational polypharmacology in modern drug discovery
Abstract
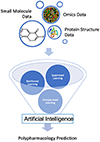
Introduction: In recent years, computational polypharmacology has gained significant attention to study the promiscuous nature of drugs. Despite tremendous challenges, community-wide efforts have led to a variety of novel approaches for predicting drug polypharmacology. In particular, some rapid advances using machine learning and artificial intelligence have been reported with great success.
Areas covered: In this article, the authors provide a comprehensive update on the current state-of-the-art polypharmacology approaches and their applications, focusing on those reports published after our 2017 review article. The authors particularly discuss some novel, groundbreaking concepts, and methods that have been developed recently and applied to drug polypharmacology studies.
Expert opinion: Polypharmacology is evolving and novel concepts are being introduced to counter the current challenges in the field. However, major hurdles remain including incompleteness of high-quality experimental data, lack of in vitro and in vivo assays to characterize multi-targeting agents, shortage of robust computational methods, and challenges to identify the best target combinations and design effective multi-targeting agents. Fortunately, numerous national/international efforts including multi-omics and artificial intelligence initiatives as well as most recent collaborations on addressing the COVID-19 pandemic have shown significant promise to propel the field of polypharmacology forward.
Keywords: Drug Polypharmacology; artificial Intelligence; deep Learning; drug Repurposing; molecular Promiscuity; multi-omics; multi-targeting Design; network Pharmacology; off-targets.
Conflict of interest statement
Declaration of Interest:
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer Disclosures:
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Figures

References
-
- Klaeger S, Heinzlmeir S, Wilhelm M, et al. The target landscape of clinical kinase drugs. Science. 2017. December 1;358(6367). doi: 10.1126/science.aan4368. - DOI - PMC - PubMed
-
A thorough analysis using chemical proteomics of the target spectrum of clinically evaluated kinase drugs and their unknown targets, offering a perspective on the “druggable” kinome, (non)kinase off-targets, and their potential therapeutic applications.
-
- Anighoro A, Bajorath J, Rastelli G. Polypharmacology: challenges and opportunities in drug discovery. J Med Chem. 2014. October 9;57(19):7874–87. doi: 10.1021/jm5006463. - DOI - PubMed
-
An excellent review on polypharmacology that describes advantages and disadvantages of combination therapy, multi-targeted drugs, and drug repurposing. It also describes applications of polypharmacology in cancer therapy and CNS diseases.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
