Effect of Antihypertensive Medication Reduction vs Usual Care on Short-term Blood Pressure Control in Patients With Hypertension Aged 80 Years and Older: The OPTIMISE Randomized Clinical Trial
- PMID: 32453368
- PMCID: PMC7251449
- DOI: 10.1001/jama.2020.4871
Effect of Antihypertensive Medication Reduction vs Usual Care on Short-term Blood Pressure Control in Patients With Hypertension Aged 80 Years and Older: The OPTIMISE Randomized Clinical Trial
Abstract
Importance: Deprescribing of antihypertensive medications is recommended for some older patients with polypharmacy and multimorbidity when the benefits of continued treatment may not outweigh the harms.
Objective: This study aimed to establish whether antihypertensive medication reduction is possible without significant changes in systolic blood pressure control or adverse events during 12-week follow-up.
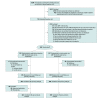
Design, setting, and participants: The Optimising Treatment for Mild Systolic Hypertension in the Elderly (OPTIMISE) study was a randomized, unblinded, noninferiority trial conducted in 69 primary care sites in England. Participants, whose primary care physician considered them appropriate for medication reduction, were aged 80 years and older, had systolic blood pressure lower than 150 mm Hg, and were receiving at least 2 antihypertensive medications were included. Participants enrolled between April 2017 and September 2018 and underwent follow-up until January 2019.
Interventions: Participants were randomized (1:1 ratio) to a strategy of antihypertensive medication reduction (removal of 1 drug [intervention], n = 282) or usual care (control, n = 287), in which no medication changes were mandated.
Main outcomes and measures: The primary outcome was systolic blood pressure lower than 150 mm Hg at 12-week follow-up. The prespecified noninferiority margin was a relative risk (RR) of 0.90. Secondary outcomes included the proportion of participants maintaining medication reduction and differences in blood pressure, frailty, quality of life, adverse effects, and serious adverse events.
Results: Among 569 patients randomized (mean age, 84.8 years; 276 [48.5%] women; median of 2 antihypertensive medications prescribed at baseline), 534 (93.8%) completed the trial. Overall, 229 (86.4%) patients in the intervention group and 236 (87.7%) patients in the control group had a systolic blood pressure lower than 150 mm Hg at 12 weeks (adjusted RR, 0.98 [97.5% 1-sided CI, 0.92 to ∞]). Of 7 prespecified secondary end points, 5 showed no significant difference. Medication reduction was sustained in 187 (66.3%) participants at 12 weeks. Mean change in systolic blood pressure was 3.4 mm Hg (95% CI, 1.1 to 5.8 mm Hg) higher in the intervention group compared with the control group. Twelve (4.3%) participants in the intervention group and 7 (2.4%) in the control group reported at least 1 serious adverse event (adjusted RR, 1.72 [95% CI, 0.7 to 4.3]).
Conclusions and relevance: Among older patients treated with multiple antihypertensive medications, a strategy of medication reduction, compared with usual care, was noninferior with regard to systolic blood pressure control at 12 weeks. The findings suggest antihypertensive medication reduction in some older patients with hypertension is not associated with substantial change in blood pressure control, although further research is needed to understand long-term clinical outcomes.
Trial registration: EudraCT Identifier: 2016-004236-38; ISRCTN identifier: 97503221.
Conflict of interest statement
Figures
Comment in
-
Deprescribing Antihypertensive Medications for Patients Aged 80 Years or Older: Is Doing Less Doing No Harm?JAMA. 2020 May 26;323(20):2024-2026. doi: 10.1001/jama.2020.4841. JAMA. 2020. PMID: 32453349 No abstract available.
-
Deprescribing Antihypertensive Medication in Elderly Adults.JAMA. 2020 Oct 27;324(16):1682. doi: 10.1001/jama.2020.16438. JAMA. 2020. PMID: 33107935 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

