Comparison of 3 Treatment Strategies for Medication Overuse Headache: A Randomized Clinical Trial
- PMID: 32453406
- PMCID: PMC7251504
- DOI: 10.1001/jamaneurol.2020.1179
Comparison of 3 Treatment Strategies for Medication Overuse Headache: A Randomized Clinical Trial
Abstract
Importance: Medication overuse headache (MOH) is a disabling, globally prevalent disorder representing a well-known and debated clinical problem. Evidence for the most effective treatment strategy is needed.
Objective: To compare 3 treatment strategies for MOH.
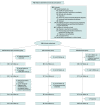
Design, setting, and participants: This open-label, randomized clinical trial with 6 months of follow-up was conducted in the tertiary sector at the Danish Headache Center, Glostrup, from October 25, 2016, to June 28, 2019. Of 483 patients with MOH referred during the inclusion period, 195 met the criteria consisting of migraine and/or tension-type headache, 18 years or older, eligibility for outpatient treatment, no severe physical or psychiatric disorder, no other addiction, and not pregnant or breastfeeding. Of these, 75 refused participation and 120 were included. Data were analyzed from July 3 to September 6, 2019.
Interventions: Random assignment (1:1:1 allocation) to 1 of the 3 outpatient treatments consisting of (1) withdrawal plus preventive treatment, (2) preventive treatment without withdrawal, or (3) withdrawal with optional preventive treatment 2 months after withdrawal.
Main outcomes and measures: The primary outcome was change in headache days per month after 6 months. Predefined secondary outcomes were change in monthly migraine days, use of short-term medication, pain intensity, number of responders, patients with remission to episodic headache, and cured MOH.
Results: Of 120 patients, 102 (mean [SD] age, 43.9 [11.8] years; 81 women [79.4%]) completed the 6-month follow-up. Headache days per month were reduced by 12.3 (95% CI, 9.3-15.3) in the withdrawal plus preventive group, by 9.9 (95% CI, 7.2-12.6) in the preventive group, and by 8.5 (95% CI, 5.6-11.5) in the withdrawal group (P = .20). No difference was found in reduction of migraine days per month, use of short-term medication, or headache intensity. In the withdrawal plus preventive group, 23 of 31 patients (74.2%) reverted to episodic headache, compared with 21 of 35 (60.0%) in the preventive group and 15 of 36 (41.7%) in the withdrawal group (P = .03). Moreover, 30 of 31 patients (96.8%) in the withdrawal plus preventive group were cured of MOH, compared with 26 of 35 (74.3%) in the preventive group and 32 of 36 (88.9%) in the withdrawal group (P = .03). These findings corresponded to a 30% (relative risk, 1.3; 95% CI, 1.1-1.6) increased chance of MOH cure in the withdrawal plus preventive group compared with the preventive group (P = .03).
Conclusion and relevance: All 3 treatment strategies were effective, but based on these findings, withdrawal therapy combined with preventive medication from the start of withdrawal is recommended as treatment for MOH.
Trial registration: ClinicalTrials.gov Identifier: NCT02993289.
Conflict of interest statement
Figures

References
-
- Global Burden of Disease Study 2013 Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386(9995):743-800. doi:10.1016/S0140-6736(15)60692-4 - DOI - PMC - PubMed
-
- Global GBD; GBD 2015 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1545-1602. doi:10.1016/S0140-6736(16)31678-6 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

