Linezolid-resistant Enterococcus faecium strains isolated from one hospital in Poland -commensals or hospital-adapted pathogens?
- PMID: 32453777
- PMCID: PMC7250452
- DOI: 10.1371/journal.pone.0233504
Linezolid-resistant Enterococcus faecium strains isolated from one hospital in Poland -commensals or hospital-adapted pathogens?
Abstract
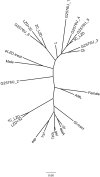
One of the most pressing problems of enterococci infections is occurring resistance to linezolid, which is an antibiotic used in the treatment of infections caused by vancomycin-resistant strains (VRE). The main objective of our research was to investigate the relationship of 19 linezolid-resistant E. faecium isolates from 18 patients hospitalized at Clinical Hospital in Gdansk (Poland). One of the LZDREF was isolated in 2003 (K2003), and another 18 were collected from 2013 to 2017. Genotyping with PCR MP method indicated 14 main unrelated genetic profiles and no association with K2003 strain. Two isolates with the same genotype and genetically closely related two sub-types (2 isolates for each sub-type) were hospital-derived colonizations of patients. The other unrelated genotypes were discussed in the context of colonization, nosocomial infections, and commensal origin, taking into account prior exposure to linezolid. We determined the presence of a point mutation G2576T in six loci of 23S rDNA. There was also a significant correlation (p<0.0015) between the presence of MIC>32 value and the presence of G2576T point mutation on the sixth rrn. We also detected 5 virulence genes for all isolates: gelE, cylA, asa1, hyl, esp. Correlation (p≤0.0001) was observed between the presence of gelE gene encoding gelatinase and two other genes: cylA and asa1 encoding cytolysin and collagen binding protein responsible for aggregation of bacterial cells, respectively. Significant correlation was also observed between asa1 and cfr genes encoding 23S rRNA rybonuclease responsible for resistance to PhLOPSA antibiotics (p = 0.0004). The multidimensional analysis has also shown the correlation between cfr gene and GI-tract (p = 0, 0491), which suggests horizontal gene transfer inside the gut microbiota and the risk of colonization with linezolid-resistant strains without previously being treated with the antibiotic. The patient could have been colonized with LZDRVREF strains which in the absence of competitive microbiota quickly settle in ecological niches favourable for them and pose a risk for the patient.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Sievert DM, Ricks P, Edwards JR, Schneider A, Patel J, Srinivasan A, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009–2010. Infect Control Hosp Epidemiol. 2013;34: 1–14. 10.1086/668770 - DOI - PubMed
-
- Mancini A, Verdini D, La Vigna G, Recanatini C, Lombardi FE, Barocci S. Retrospective analysis of nosocomial infections in an Italian tertiary care hospital. New Microbiol. 2016;39(3): 197–205. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

