Methodology for the American Society of Hematology VTE guidelines: current best practice, innovations, and experiences
- PMID: 32453843
- PMCID: PMC7252554
- DOI: 10.1182/bloodadvances.2020001768
Methodology for the American Society of Hematology VTE guidelines: current best practice, innovations, and experiences
Abstract
Background: Methods for the development of clinical guidelines have advanced dramatically over the past 2 decades to strive for trustworthiness, transparency, user-friendliness, and rigor. The American Society of Hematology (ASH) guidelines on venous thromboembolism (VTE) have followed these advances, together with application of methodological innovations.
Objective: In this article, we describe methods and methodological innovations as a model to inform future guideline enterprises by ASH and others to achieve guideline standards. Methodological innovations introduced in the development of the guidelines aim to address current challenges in guideline development.
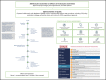
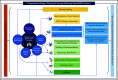
Methods: We followed ASH policy for guideline development, which is based on the Guideline International Network (GIN)-McMaster Guideline Development Checklist and current best practices. Central coordination, specialist working groups, and expert panels were established for the development of 10 VTE guidelines. Methodological guidance resources were developed to guide the process across guidelines panels. A methods advisory group guided the development and implementation of methodological innovations to address emerging challenges and needs.
Results: The complete set of VTE guidelines will include >250 recommendations. Methodological innovations include the use of health-outcome descriptors, online voting with guideline development software, modeling of pathways for diagnostic questions, application of expert evidence, and a template manuscript for publication of ASH guidelines. These methods advance guideline development standards and have already informed other ASH guideline projects.
Conclusions: The development of the ASH VTE guidelines followed rigorous methods and introduced methodological innovations during guideline development, striving for the highest possible level of trustworthiness, transparency, user-friendliness, and rigor.
Conflict of interest statement
Conflict-of-interest disclosure: A.C. has served as a consultant for Synergy and CRO, and received institutional research support on his behalf from Alexion, Bayer, Novo Nordisk, Pfizer, Sanofi, Spark, and Takeda. A.R. has served on an advisory board for Alexion, Baxter, Bayer, Kedrion Biopharma, and Octapharma Plasma, and has received institutional research support on her behalf from Alnylam (Sanofi Genzyme), Baxalta (Shire), Biomarin, Dimensions Therapeutics, Genetech, Janssen Pharmaceuticals, and Roche. D.M.W. has served as a consultant for, and received institutional research support on his behalf from, Roche Diagnostics. E.A.A. has served as a guideline methodologist on other guidelines related to VTE, and has led a number of systematic reviews on VTE. G.H.L. is a principal investigator on a research grant from Amgen to the Fred Hutchinson Cancer Research Center, and has consulted within the past 2 years for the following companies on other topics: Biotheranostics, Beyond Spring, G1 Therapeutics, Invitae, Mylan, Partners Therapeutics, Samsung, and Spectrum. H.J.S. was cochair of the ASH VTE guidelines and is cochair of the GRADE Working Group; has developed methods, tools, and approaches for guideline development; has coauthored the background papers for the IOM report on trustworthy guidelines, the GIN-McMaster guideline development checklist, and other tools; is a consultant to ministries of health, the World Health Organization, and professional societies on guideline methodology; and is director of Cochrane Canada (Cochrane methods have served for this guideline). R.K. and K.E.A. are employed by ASH, which has funded and disseminated the guidelines discussed in this paper. S.M.B. reports honoraria from Leo Pharma. S.M. reports research grants and honoraria from Aspen, Bayer, Bristol-Myers Squibb–Pfizer, Boehringer Ingelheim, Daiichi Sankyo, Pfizer, Portola, and Sanofi; all fees were paid to the author’s institution. T.L.O. has served as a consultant for Instrumentation Laboratory, and has received institutional research support on his behalf from Instrumentation Laboratory, Siemens, Stago, the National Institutes of Health, the Centers for Disease Control and Prevention, and the Patient-Centered Outcomes Research Institute. The remaining authors declare no competing financial interests.
Figures






References
-
- Qaseem A, Forland F, Macbeth F, Ollenschläger G, Phillips S, van der Wees P; Board of Trustees of the Guidelines International Network. Guidelines International Network: toward international standards for clinical practice guidelines. Ann Intern Med. 2012;156(7):525-531. - PubMed
-
- Institute of Medicine of the National Academies Committee on Standards for Developing Trustworthy Clinical Practice Guidelines; Graham R, Mancher M, Miller Wolman D, Greenfield S, Steinberg E, eds. Clinical Practice Guidelines We Can Trust. Washington, DC: The National Academies Press; 2011. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

