Evidence for Simultaneous Dearomatization of Two Aromatic Rings under Mild Conditions in Cu(I)-Catalyzed Direct Asymmetric Dearomatization of Pyridine
- PMID: 32453952
- PMCID: PMC8171382
- DOI: 10.1021/jacs.0c04486
Evidence for Simultaneous Dearomatization of Two Aromatic Rings under Mild Conditions in Cu(I)-Catalyzed Direct Asymmetric Dearomatization of Pyridine
Abstract
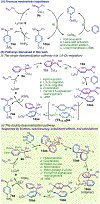
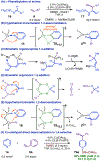
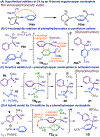
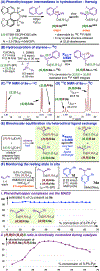
Bis(phosphine) copper hydride complexes are uniquely able to catalyze direct dearomatization of unactivated pyridines with carbon nucleophiles, but the mechanistic basis for this result has been unclear. Here we show that, contrary to our initial hypotheses, the catalytic mechanism is monometallic and proceeds via dearomative rearrangement of the phenethylcopper nucleophile to a Cpara-metalated form prior to reaction at heterocycle C4. Our studies support an unexpected heterocycle-promoted pathway for this net 1,5-Cu-migration beginning with a doubly dearomative imidoyl-Cu-ene reaction. Kinetics, substituent effects, computational modeling, and spectroscopic studies support the involvement of this unusual process. In this pathway, the CuL2 fragment subsequently mediates a stepwise Cope rearrangement of the doubly dearomatized intermediate to the give the C4-functionalized 1,4-dihydropyridine, lowering a second barrier that would otherwise prohibit efficient asymmetric catalysis.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



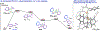


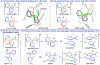


References
-
-
For reviews on pyridine dearomatization and dihydropyridine chemistry, see:
- Lavilla R Recent Developments in the Chemistry of Dihydropyridines. J. Chem. Soc., Perkin Trans 1, 2002, 1141–1156.
- Ahamed M; Todd MH Catalytic Asymmetric Additions of Carbon-Centered Nucleophiles to Nitrogen-Containing Aromatic Heterocycles. Eur. J. Org. Chem 2010, 2010, 5935–5942.
- Bull JA; Mousseau JJ; Pelletier G; Charette AB Synthesis of Pyridine and Dihydropyridine Derivatives by Regio- and Stereoselective Addition to N-Activated Pyridines. Chem. Rev 2012, 112, 2642–2713. - PubMed
- Zhuo C-X; Zhang W; You S-L Catalytic Asymmetric Dearomatization Reactions. Angew. Chem. Int. Ed 2012, 51, 12662–12686. - PubMed
- Ding Q; Zhou X; Fan R Recent Advances in Dearomatization of Heteroaromatic Compounds. Org. Biomol. Chem 2014, 12, 4807–4815. - PubMed
- Bertuzzi G; Bernardi L; Fochi M Nucleophilic Dearomatization of Activated Pyridines. Catalysts 2018, 8, 632–665.
-
-
- Vitaku E; Smith DT; Njardarson JT Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem 2014, 57, 10257–10274. - PubMed
-
- Comins DL; Abdullah AH Regioselective Addition of Grignard Reagents to 1-Acylpyridinium Salts. A Convenient Method for the Synthesis of 4-Alkyl(aryl)pyridines. J. Org. Chem 1982, 47, 4315–4319.
-
-
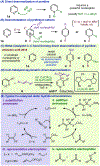
Reports on catalyzed direct addition of hydride or silyl nucleophiles to pyridine had appeared in preceding years and some are conceptually related to the Cu-catalyzed asymmetric dearomatization; the reaction with styrenes in Figure 1, D, can be viewed formally as a diverted 1,4-hydrosilylation of pyridine in which the hydride is intercepted by an olefin to generate a carbon-centered nucleophile rather than undergoing attack at heterocycle C4. See
- Oshima K; Ohmura T; Suginome M Palladium-Catalyzed Regioselective Silaboration of Pyridines Leading to the Synthesis of Silylated Dihydropyridines. J. Am. Chem. Soc 2011, 133, 7324–7327. - PubMed
- Gutsulyak DV; van der Est A; Nikonov GI Facile Catalytic Hydrosilylation of Pyridines. Angew. Chem. Int. Ed 2011, 50, 1384–1387. - PubMed
- Hill MS; Kociok-Köhn G; MacDougall DJ; Mahon MF; Weetman C Magnesium Hydrides and the Dearomatisation of Pyridine and Quinoline derivatives. Dalton Trans 2011, 40, 12500–12509. - PubMed
- Königs CDF; Klare HFT; Oestreich M Catalytic 1,4-Selective Hydrosilylation of Pyridines and Benzannulated Congeners. Angew. Chem. Int. Ed 2013, 52, 10076–10079. - PubMed
- Dudnik AS; Weidner VL; Motta A; Delferro M; Marks TJ Nat. Chem 2014, 6, 1100–1107. - PubMed
- Gandhamsetty N; Park S; Chang S Atom-Efficient Regioselective 1,2-Dearomatization of Functionalized Pyridines by an Earth-Abundant Organolanthanide Catalyst. J. Am. Chem. Soc 2015, 137, 15176–15184.
- Intemann J; Bauer H; Pahl J; Maron L; Harder S Calcium Hydride Catalyzed Highly 1,2-Selective Pyridine Hydrosilylation. Chem. Eur. J 2015, 21, 11452–11461. - PubMed
- Fan X; Zheng J; Li ZH; Wang H Organoborane Catalyzed Regioselective 1,4-Hydroboration of Pyridines. J. Am. Chem. Soc 2015, 137, 4916–4919. - PubMed
- Kaithal A; Chatterjee B; Gunanathan C Ruthenium-Catalyzed Regioselective 1,4-Hydroboration of Pyridines. Org. Lett 2016, 18, 3402–3405. - PubMed
- Zhang F; Song H; Zhuang X; Tung C-H; Wang W Iron-Catalyzed 1,2-Selective Hydroboration of N-Heteroarenes. J. Am. Chem. Soc, 2017, 139, 17775–17778. - PubMed
- Yu H-C; Islam SM; Mankad NP Cooperative Heterobimetallic Substrate Activation Enhances Catalytic Activity and Amplifies Regioselectivity in 1,4-Hydroboration of Pyridines. ACS Catal 2020, 10, 3670–3675.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

