The evolution of host resistance and parasite infectivity is highest in seasonal resource environments that oscillate at intermediate amplitudes
- PMID: 32453992
- PMCID: PMC7287369
- DOI: 10.1098/rspb.2020.0787
The evolution of host resistance and parasite infectivity is highest in seasonal resource environments that oscillate at intermediate amplitudes
Abstract
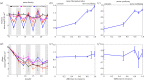
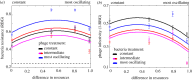
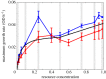
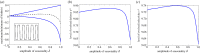
Seasonal environments vary in their amplitude of oscillation but the effects of this temporal heterogeneity for host-parasite coevolution are poorly understood. Here, we combined mathematical modelling and experimental evolution of a coevolving bacteria-phage interaction to show that the intensity of host-parasite coevolution peaked in environments that oscillate in their resource supply with intermediate amplitude. Our experimentally parameterized mathematical model explains that this pattern is primarily driven by the ecological effects of resource oscillations on host growth rates. Our findings suggest that in host-parasite systems where the host's but not the parasite's population growth dynamics are subject to seasonal forcing, the intensity of coevolution will peak at intermediate amplitudes but be constrained at extreme amplitudes of environmental oscillation.
Keywords: Pseudomonas fluorescens; adaptive dynamics; coevolution; host–parasite; mathematical modelling; oscillating environment.
Conflict of interest statement
We declare we have no competing interests.
Figures
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
