Self-assembling prodrug nanotherapeutics for synergistic tumor targeted drug delivery
- PMID: 32454086
- PMCID: PMC7245299
- DOI: 10.1016/j.actbio.2020.05.026
Self-assembling prodrug nanotherapeutics for synergistic tumor targeted drug delivery
Abstract
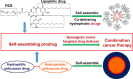
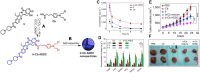
Self-assembling prodrugs represents a robust and effective nanotherapeutic approach for delivering poorly soluble anticancer drugs. With numerous intrinsic advantages, self-assembling prodrugs possess the maximum drug loading capacity, controlled drug release kinetics, prolonged blood circulation, and preferential tumor accumulation based on the enhanced permeability and retention (EPR) effect. These prodrug conjugates allow for efficient self-assembly into nanodrugs with the potential of encapsulating other therapeutic agents that have different molecular targets, enabling simultaneous temporal-spatial release of drugs for synergistic antitumor efficacy with reduced systemic side effects. The aim of this review is to summarize the recent progress of self-assembling prodrug cancer nanotherapeutics that are made through conjugating therapeutically active agents to Polyethylene glycol, Vitamin E, or drugs with different physicochemical properties via rational design, for synergistic tumor targeted drug delivery. STATEMENT OF SIGNIFICANCE: All current FDA-approved nanomedicines use inert biomaterials as drug delivery carriers. These biomaterials lack any therapeutic potential, contributing not only to the cost, but may also elicit severe unfavorable adverse effects. Despite the reduction in toxicity associated with the payload, these nanotherapeutics have been met with limited clinical success, likely due to the monotherapy regimen. The self-assembling prodrug (SAP) has been emerging as a powerful platform for enhancing efficacy through co-delivering other therapeutic modalities with distinct molecular targets. Herein, we opportunely present a comprehensive review article summarizing three unique approaches of making SAP for synergistic drug delivery: pegylation, vitamin E-derivatization, and drug-drug conjugation. These SAPs may inevitably pave the way for developing more efficacious, clinically translatable, combination cancer nanotherapies.
Keywords: Enhanced Cancer Therapy; Nanotherapeutics; Self-assembling prodrug; Synergistic drug delivery.
Copyright © 2020. Published by Elsevier Ltd.
Conflict of interest statement
Declaration of Competing Interest There are no conflicts of interest to declare.
Figures


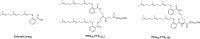

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

