Immune checkpoint inhibitor-related dermatologic adverse events
- PMID: 32454097
- PMCID: PMC7572894
- DOI: 10.1016/j.jaad.2020.03.132
Immune checkpoint inhibitor-related dermatologic adverse events
Abstract
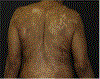
Immune checkpoint inhibitors have emerged as a pillar in the management of advanced malignancies. However, nonspecific immune activation may lead to immune-related adverse events, wherein the skin and its appendages are the most frequent targets. Cutaneous immune-related adverse events include a diverse group of inflammatory reactions, with maculopapular rash, pruritus, psoriasiform and lichenoid eruptions being the most prevalent subtypes. Cutaneous immune-related adverse events occur early, with maculopapular rash presenting within the first 6 weeks after the initial immune checkpoint inhibitor dose. Management involves the use of topical corticosteroids for mild to moderate (grades 1-2) rash, addition of systemic corticosteroids for severe (grade 3) rash, and discontinuation of immunotherapy with grade 4 rash. Bullous pemphigoid eruptions, vitiligo-like skin hypopigmentation/depigmentation, and psoriasiform rash are more often attributed to programmed cell death-1/programmed cell death ligand-1 inhibitors. The treatment of bullous pemphigoid eruptions is similar to the treatment of maculopapular rash and lichenoid eruptions, with the addition of rituximab in grade 3-4 rash. Skin hypopigmentation/depigmentation does not require specific dermatologic treatment aside from photoprotective measures. In addition to topical corticosteroids, psoriasiform rash may be managed with vitamin D3 analogues, narrowband ultraviolet B light phototherapy, retinoids, or immunomodulatory biologic agents. Stevens-Johnson syndrome and other severe cutaneous immune-related adverse events, although rare, have also been associated with checkpoint blockade and require inpatient care as well as urgent dermatology consultation.
Keywords: CTLA-4 inhibitor; PD-1 inhibitor; PD-L1 inhibitor; checkpoint inhibitor; dermatologic adverse event; immune-related cutaneous adverse event; lichenoid eruption; maculopapular rash; pruritus; vitiligo.
Copyright © 2020 American Academy of Dermatology, Inc. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflicts of interest: Dr Kern has scientific advisory board roles with Oncimmune and Biodesix and consultant roles with Uptake Medical and Cireca. Dr Lacouture has consultant/speaking roles with ADC Therapeutics America, Inc, Apricity Health, LLC, Azitra, Inc, Deciphera, Johnson and Johnson, NCODA, Novocure Inc, Kyowa Kirin, Inc, Janssen Research and Development LLC, Menlo Therapeutics, Novartis Pharmaceuticals Corp, QED Therapeutics, F. Hoffmann-La Roche AG, Amgen Inc, Astrazeneca Pharmaceuticals LP, Genentech Inc, Seattle Genetics, Lutris, Paxman Coolers, Teva Mexico, Parexel, OnQuality Pharmaceuticals Ltd, Oncodermatology, and Takeda Millenium and receives research funding from Lutris, Paxman, Novocure Inc, US Biotest, and Veloce. Ms Geisler, Mr Phillips, Ms Barrios, and Drs Wu, Leung, and Moy have no conflicts of interest to disclose.
Figures







Comment in
-
Limitations of morphology-based management for immune checkpoint inhibitor-related cutaneous adverse events.J Am Acad Dermatol. 2021 Jun;84(6):e281-e282. doi: 10.1016/j.jaad.2021.01.054. Epub 2021 Jan 23. J Am Acad Dermatol. 2021. PMID: 33493573 No abstract available.
-
Reply to "Immune checkpoint inhibitor-related dermatologic adverse events".J Am Acad Dermatol. 2021 Jun;84(6):e297-e298. doi: 10.1016/j.jaad.2021.02.064. Epub 2021 Mar 1. J Am Acad Dermatol. 2021. PMID: 33662463 No abstract available.
References
-
- Inno A, Metro G, Bironzo P, et al. Pathogenesis, clinical manifestations and management of immune checkpoint inhibitors toxicity. Tumori. 2017;103:405–421. - PubMed
-
- Lacouture ME, Wolchok JD, Yosipovitch G, Kahler KC, Busam KJ, Hauschild A. Ipilimumab in patients with cancer and the management of dermatologic adverse events. J Am Acad Dermatol. 2014;71:161–169. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

