Review of trials currently testing treatment and prevention of COVID-19
- PMID: 32454187
- PMCID: PMC7245266
- DOI: 10.1016/j.cmi.2020.05.019
Review of trials currently testing treatment and prevention of COVID-19
Erratum in
-
Corrigendum to "Review of trials currently testing treatment and prevention of COVID-19" [Clin Microbiol Infect 26.8 (2020) 988-998].Clin Microbiol Infect. 2021 Mar;27(3):499. doi: 10.1016/j.cmi.2021.01.024. Epub 2021 Feb 2. Clin Microbiol Infect. 2021. PMID: 33540115 Free PMC article. No abstract available.
Abstract
Background: As COVID-19 cases continue to rise globally, evidence from large randomized controlled trials is still lacking. Currently, numerous trials testing potential treatment and preventative options are being undertaken all over the world.
Objectives: We summarized all registered clinical trials examining treatment and prevention options for COVID-19. Additionally, we evaluated the quality of the retrieved studies.
Data sources: Clinicaltrials.gov, the Chinese Clinical Trial Registry and the European Union Clinical Trials Register were systematically searched.
Study eligibility criteria: Registered clinical trials examining treatment and/or prevention options for COVID-19 were included. No language, country or study design restrictions were applied. We excluded withdrawn or cancelled studies and trials not reporting therapeutic or preventative strategies for COVID-19.
Participants and interventions: No restrictions in terms of participants' age and medical background or type of intervention were enforced.
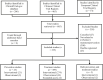
Methods: The registries were searched using the term 'coronavirus' or 'COVID-19' from their inception until 26 March 2020. Additional manual search of the registries was also performed. Eligible studies were summarized and tabulated. Interventional trials were methodologically analysed, excluding expanded access studies and trials testing traditional Chinese medicine.
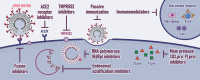
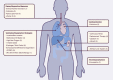
Results: In total, 309 trials evaluating therapeutic management options, 23 studies assessing preventive strategies and three studies examining both were retrieved. Finally, 214 studies were methodologically reviewed. Interventional treatment studies were mostly randomized (n = 150/198, 76%) and open label (n = 73/198, 37%) with a median number of planned inclusions of 90 (interquartile range 40-200). Major categories of interventions that are currently being investigated are discussed.
Conclusions: Numerous clinical trials have been registered since the onset of the COVID-19 pandemic. Summarized data on these trials will assist physicians and researchers to promote patient care and guide future research efforts for COVID-19 pandemic containment.
Keywords: Anti-inflammatory; Antivirals; COVID-19; Clinical trials; Immunomodulators; Novel coronavirus; Pneumonia; Prevention; SARS-CoV-2; Treatment.
Copyright © 2020 European Society of Clinical Microbiology and Infectious Diseases. Published by Elsevier Ltd. All rights reserved.
Figures


References
-
- Home – ClinicalTrials.gov. https://clinicaltrials.gov/ n.d.
-
- Chinese Clinical Trial Register (ChiCTR) The world health organization international clinical trials registered organization registered platform. http://www.chictr.org.cn/searchprojen.aspx n.d.
-
- EU clinical trials register. https://www.clinicaltrialsregister.eu/ Update n.d.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

