Light Quenching and Fluorescence Depolarization of Rhodamine B and Applications of This Phenomenon to Biophysics
- PMID: 32454536
- PMCID: PMC7243262
- DOI: 10.1021/j100052a055
Light Quenching and Fluorescence Depolarization of Rhodamine B and Applications of This Phenomenon to Biophysics
Abstract
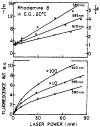
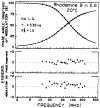
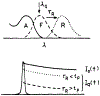
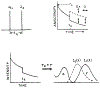
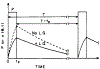
The fluorescence intensity of rhodamine B (RhB) was found to display a sublinear dependence on incident power when excited with the focused output of a cavity-dumped dye laser. This effect was found to be proportional to the amplitude of the emission spectrum at the incident wavelength and to be associated with a decrease in the time-zero anisotropy of RhB. The absence of changes in the intensity decay law or rotational correlation time indicates the absence of photochemical processes. These results are consistent with "light quenching" of RhB due to stimulated emission. In viscous solution the extent of depolarization of the emission was found to be in agreement with theoretical expressions which account for photoselective light quenching and for spatial inhomogeneities in the incident laser beam. The phenomenon of light quenching has numerous potential applications in biophysics, such as studies of the orientation and dynamics of fluorescent macromolecules.
Figures
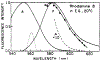





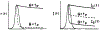


References
-
- Winnik MA,, Ed. Photophysical and Photochemical Tools in Polymer Science; D. Reidel Publishing Co.: Dordrecht, Holland, 1986.
-
- Warner IM, McGown LB, Eds. Advances in Multidimensional Luminescence; JAI Press, Inc.: Greenwich, CT, 1991; Vol. 1.
-
- Fleming GR Chemical Applications of Ultrafast Spectroscopy; Oxford University Press: New York, 1986.
-
- Dewey TG Biophysical and Biochemical Aspects of Fluorescence Spectroscopy; Plenum Press: New York, 1991.
-
- Lakowicz JR,Ed. Time-Resoled Laser Spectroscopy in Biochemistry III Proceedings of SPIE;SPIE Bellingham, WA, 1992: Vol. 1640.
Grants and funding
LinkOut - more resources
Full Text Sources
