Development and Validation of a Stability Indicating RP-HPLC Method for Simultaneous Estimation of Teneligliptin and Metformin
- PMID: 32454773
- PMCID: PMC7227910
- DOI: 10.4274/tjps.galenos.2018.16768
Development and Validation of a Stability Indicating RP-HPLC Method for Simultaneous Estimation of Teneligliptin and Metformin
Abstract
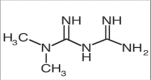
Objectives: The main objective of the present work is to develop a simple, precise, specific and stability method indicating reverse phase high performance liquid chromatography method for simultaneous estimation of teneligliptin and metformin in bulk and tablet dosage form.
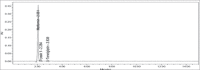
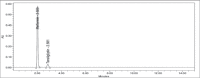
Materials and methods: The analysis was performed with a Kromasil C18 column (250×4.6 mm, 5 μm) at 30°C using buffer: acetonitrile: methanol (65:25:10, v/v/v) as mobile phase. The detection was carried out with a flow rate of 1.0 mL/min at 254 nm.
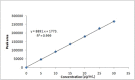
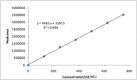
Results: The retention time of teneligliptin and metformin was 2.842 min and 2.017 min, respectively. The linearity range was 5-30 μg/mL for teneligliptin and 125-750 μg/mL for metformin. The forced degradation studies were performed as per the guidelines of the The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use under acidic, alkaline, oxidative, thermal, photostability, and neutral conditions.
Conclusion: This method was successfully validated for all the parameters and could detect the the correct amounts of active drug substance in formulations that are available in the market. This developed method in the present study could be successfully employed for the simultaneous estimation of teneligliptin and metformin in bulk and tablet dosage form.
Keywords: RP-HPLC; Teneligliptin; metformin; stability studies; validation.
©Copyright 2020 Turk J Pharm Sci, Published by Galenos Publishing House.
Conflict of interest statement
Conflicts of interest: No conflict of interest was declared by the authors. The authors alone are responsible for the content and writing of this article.
Figures





Similar articles
-
Stability-indicating HPLC method development and validation for simultaneous estimation of metformin, dapagliflozin, and saxagliptin in bulk drug and pharmaceutical dosage form.Biomed Chromatogr. 2022 Jul;36(7):e5384. doi: 10.1002/bmc.5384. Epub 2022 Apr 29. Biomed Chromatogr. 2022. PMID: 35434817
-
Development and Validation of Stability-indicating HPLC Method for Simultaneous Estimation of Cefixime and Linezolid.Indian J Pharm Sci. 2014 Nov-Dec;76(6):535-40. Indian J Pharm Sci. 2014. PMID: 25593387 Free PMC article.
-
Stability-Indicating RP-HPLC Method for Simultaneous Estimation of Enrofloxacin and Its Degradation Products in Tablet Dosage Forms.J Anal Methods Chem. 2015;2015:735145. doi: 10.1155/2015/735145. Epub 2015 Jan 29. J Anal Methods Chem. 2015. PMID: 25705547 Free PMC article.
-
Stability-indicating ultra performance liquid chromatography method development and validation for simultaneous estimation of metformin, linagliptin, and empagliflozin in bulk and pharmaceutical dosage form.Biomed Chromatogr. 2021 Apr;35(4):e5019. doi: 10.1002/bmc.5019. Epub 2020 Dec 9. Biomed Chromatogr. 2021. PMID: 33146442
-
Analytical Methods for Estimating Metformin Hydrochloride, Pioglitazone and Teneligliptin in Diabetes Management: a Comprehensive Review.J Chromatogr Sci. 2025 Feb 8;63(3):bmaf013. doi: 10.1093/chromsci/bmaf013. J Chromatogr Sci. 2025. PMID: 40037341 Review.
Cited by
-
A Facile Method for Screening DPP IV Inhibitors in Living Cell System Based on Enzyme Activity Probe.J Anal Methods Chem. 2025 Jun 16;2025:1616740. doi: 10.1155/jamc/1616740. eCollection 2025. J Anal Methods Chem. 2025. PMID: 40551829 Free PMC article.
-
A ratiometric fluorescent sensor for detection of metformin based on terbium-1,10-phenanthroline-nitrogen-doped-graphene quantum dots.RSC Adv. 2022 Aug 10;12(34):22255-22265. doi: 10.1039/d2ra02611b. eCollection 2022 Aug 4. RSC Adv. 2022. PMID: 36043095 Free PMC article.
-
Development and Validation of a Stability-Indicating RP-HPLC Method for the Simultaneous Estimation of Bictegravir, Emtricitabine, and Tenofovir Alafenamide Fumarate.Turk J Pharm Sci. 2021 Sep 1;18(4):410-419. doi: 10.4274/tjps.galenos.2020.70962. Turk J Pharm Sci. 2021. PMID: 34496481 Free PMC article.
References
-
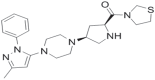
- Yoshida T, Akhashi F, Sakashita H, Kitajima H, Nakamura M, Sonda S, Takeuchi M, Tanaka Y, Ueda N, Sekiguchi S, Ishige T, Shima K, Nabeno M, Abe Y, Anabuki J, Soejima A, Yoshida K, Takashina Y, Ishii S, Kiuchi S, Fukuda S, Tsutsumiuchi R, Kosaka K, Murozono T, Nakamaru Y, Utsumi H, Masutomi N, Kishida H, Miyaguchi I, Hayashi Y. Discovery and preclinical profile of teneligliptin (3-[(2S,4S)-4-[4-(3-methyl-1-phenyl-1H-pyrazol-5-yl)piperazin-1-yl]pyrrolidin-2-ylcarbonyl]thiazolidine): a highly potent, selective, long-lasting and orally active dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes. Bioorg Med Chem. 2012;20:5705–5719. - PubMed
-
- No authors listed. Indian Pharmacopoeia, Government of India, Ministry of Health & Family Welfare, Volume-2. Ghaziabad; the Indian Pharmacopoeia Commission. 2007:1358–1359.
-
- Merck. The Merck Index (13th ed) White House Station; Merck & Co Inc. 2001;1061.
-
- Sweetman S. ed. Martindale: The Complete Drug Reference Vol. 1. (36th ed) London; Pharmaceutical Press. 2009:453–454.
LinkOut - more resources
Full Text Sources
